

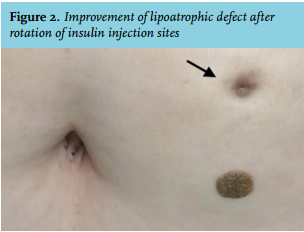
Although the patient did not allow us to perform a skin biopsy, the findings were consistent with severe necrotic insulin-induced lipoatrophy. We told her that it is mandatory to rotate injection sites and the dose of premixed insulin was decreased to 68 units daily. Four months later, her glycated haemoglobin level had decreased to 7.1%, along with marked improvement in the lipoatrophic area (figure 2).
Some patients tend to use the same injection site, since it leads to reduction of pain sensation. However, this increases the risk of dermatological complications of insulin therapy: insulin-induced lipohypertrophy and lipoatrophy.1 Lipohypertrophy is far more common, but both conditions may lead to poor glycaemic control. Insulin-induced lipoatrophy is considered to be an immune complex-mediated inflammatory lesion.1 Any insulin formulation can in principle cause lipoatrophy. The prevalence of this condition was 2.5% in patients who used older bovine and porcine insulins.2 But its prevalence has tremendously decreased with the use of newer insulin analogues.3 Only 13 cases associated with the use of lispro, aspart, glargine or detemir insulin have been reported so far.3 There are no strict guidelines for the treatment of lipoatrophy. Topical disodium cromoglycate, topical and systemic glucocorticoids have been occasionally used for the treatment of insulin-induced lipoatrophy.4 However, randomised clinical trials have not been performed to assess their true efficacy. Rotation of injection sites has the most important role in lipoatrophy treatment, as seen in our case. In conclusion, it is important for clinicians to recognise lipoatrophy as a potential complication of therapy with insulin analogues, since it may lead to impaired insulin absorption and poor glycaemic control.
INFORMED CONSENT
The patient gave her written consent for this case study.
DISCLOSURES
The authors declare that they have no conflict of interest.
This research did not receive any financial support.
REFERENCES