

KEYWORDS
Nephropathia endemica, orthohantavirus infection, puumala virus
INTRODUCTION
Orthohantaviruses compromise a group of enveloped RNA viruses of which, over 21, are associated with human disease.1 Orthohantaviruses are transmitted through rodent hosts, insectivores, and bats. Different hosts carry different orthohantaviruses. An overview of orthohantaviruses found in the Netherlands can be found in table 1 and figure 1.2-4 Puumala virus is the most common type in the Netherlands, which has been found in bank voles throughout the country and is known to cause human infections, mostly in the east, the region of Twente, and south, the province of Noord-Brabant.2,3,5 This case report describes a patient with an orthohantavirus infection in Groningen, located in the north of the Netherlands.
CASE REPORT
A 38-year-old female was admitted to our hospital in June 2019 with fever and headache since a few days. Physical examination showed no abnormalities. Laboratory analysis showed a mild renal insufficiency, a thrombocytopenia, and an elevated C-reactive protein (CRP) (table 2). Blood cultures were taken and a chest X-ray was normal. Because of persistent fever cefuroxime was started on the suspicion of a bacterial infection. The acute renal failure was considered a prerenal impairment resulting from dehydration.
One day after hospitalisation, her fever disappeared, and her headache improved under acetaminophen. Antibiotics were discontinued because of persistent negative blood cultures and lack of a proven bacterial infection. However, in contrast to clinical improvement, the patient’s renal function deteriorated (table 2). An ultrasound of the kidneys excluded postrenal obstruction. The urinalysis showed no leucocyturia or erythrocyturia but did determine a mild albuminuria. Auto-immune serology showed no abnormalities. Serology, PCR and blood culture for leptospirosis was negative (table 3). On repeated anamnesis, the patient claimed to have cleaned up faecal excrements of mice a few days earlier. The patient reported no recent traveling neither abroad nor to other areas in the Netherlands.
The patient was discharged after a renal biopsy and diagnostics for orthohantavirus on blood was performed, nine days after the first symptoms. At the time of the outpatient clinic visit, the patient’s renal function and thrombocytes had been restored to normal. Puumala virus serology turned out to be positive (both IgM and IgG). PCR and blood culture for orthohantavirus was negative. The renal biopsy specimen showed few reactive changes with little increase of monocyte infiltration in the interstitium, no signs of a tubulo-interstitial nephritis or glomerulonephritis. 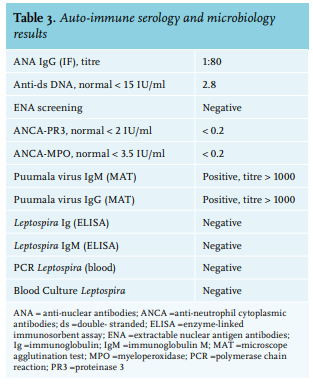
Orthohantaviruses are transmitted to humans by aerosols from contaminated excreta of infected rodents. It is thought that the type of orthohantavirus is associated with a specific host species due to co-evolution of the viruses and reservoir hosts.6 Orthohantaviruses predominantly infect endothelial cells leading to damage of the vascular barrier, which is the cornerstone of the pathophysiology.7 This can lead to three clinical syndromes: haemorrhagic fever with renal syndrome (HFRS), nephropathia epidemica (NE), and orthohantavirus cardiopulmonary syndrome (HCPS), depending on the type of orthohantavirus.1 Orthohantavirus subtypes that cause HCPS are only found in the Americas, due to the distribution of the rodent hosts.
HFRS is caused by the Seoul, Hantaan and Dobrava-Belgrade viruses. The Seoul and Hantaan viruses are transmitted by rats, and the Dobrava-Belgrade virus by the yellow-necked mouse. In the Netherlands, human HFRS cases are only caused by Seoul virus infections transmitted by brown rats.3,8 The rodent host of the Dobrava-Belgrade virus is present in the Netherlands, but this virus is not known to circulate.9,10 HFRS starts with a flu-like presentation such as high fever, headache, backache, abdominal pains, nausea, and vomiting. Later, HFRS can lead to haemorrhages such as conjunctival haemorrhages, petechiae, epistaxis, and even intracranial haemorrhages. The earliest and more specific laboratory abnormality is a rapid decline in platelet count. HFRS can lead to renal manifestations like reduced kidney function with oliguria or anuria, proteinuria, and microscopic haematuria.9 Puumala virus causes NE, a milder form of HFRS. NE is not correlated to haemorrhagic manifestations and shock.11 Our patient had flu-like symptoms preceding the decrease in kidney function, which fits with the natural course of NE.12
In the Netherlands, there is a reporting obligation for orthohantavirus since 2008. The incidence has been low (0.0-0.3 per 100,000, years 2008-2018) with a total of 62 reported autochthonous cases, most of which were Puumala virus infections in Twente.13 This is slightly lower than the average incidence in Europe overall (0.4-0.8 per 100,000, years 2013-2017). Most of these cases occurred in Finland or Germany.14 The highest incidence of orthohantavirus (HFRS) is found in China, with a reported incidence of 0.83 per 100,000.15
A study in healthy individuals in the Netherlands showed a seroprevalence of 1.7% with the highest rate of 3.2% in Twente.2 Our patient denied traveling to Twente or other countries, which makes it likely that she got infected in the Groningen area where she resides. The low number of reported cases in comparison to the relatively high seroprevalence suggests that orthohantavirus infections are underdiagnosed, likely due to the subclinical course of the disease or due to misdiagnosis. It is also possible that the infections are mistaken for leptospirosis, since symptoms have many similarities with those of NE. A Dutch study in patients with suspected leptospirosis showed that seven patients had an acute orthohantavirus infection, of which three were initially not diagnosed.16 This was also the case in our patient, where the orthohantavirus diagnosis was considered only during hospitalisation after a further thorough inquiry. If the rodent exposure had been clarified earlier, a serology test, which is an inexpensive and accessible test, could have been performed and a kidney biopsy could have been avoided.17
CONCLUSION
We report a patient with NE due to an infection with the Puumala orthohantavirus acquired in the north of the Netherlands. Reported autochthonous cases are rare, but the high seroprevalence suggests that orthohantavirus infections are underdiagnosed. Orthohantaviruses should be included in the differential diagnosis in patients with flu-like symptoms, renal failure, or thrombocytopenia, also in areas of the Netherlands where orthohantavirus infections are less common, such as in the north. A thorough exposure inquiry is essential.
DISCLOSURES
All authors declare no conflicts of interest. No funding or financial support was received.
REFERENCES