

KEYWORDS
Anticoagulants, compliance, drug monitoring guideline, low-molecular-weight heparin
INTRODUCTION
Anticoagulants are widely used in preventing and treating venous thromboembolism (VTE). Several anticoagulant therapies are available, each with different pharmacodynamic and pharmacokinetic properties. For ambulant use, the recently developed direct oral anticoagulants (DOAC) are increasingly used, mainly as an oral application; in addition, therapeutic drug monitoring (TDM) is not required, in contrast to the classically used coumarin derivates.1 Anticoagulants are also frequently used in-hospital. Hospitalised patients are classically treated with low-molecular-weight heparins (LMWH), primarily for bridging therapy during a perioperative period or during cancer-associated VTE.2,3 The applicability of coumarin derivates is limited because of its teratogenicity during pregnancy and because patients may have altered metabolism during disease in a hospital setting, resulting in unstable plasma-levels.4 DOACs could be considered an alternative, but safety and optimal use are still subject to current investigation. Moreover, tailored intervention in cases of bleeding is not yet available for all subclasses.5 For those reasons, many hospitalised patients are still treated with LMWHs.
LMWHs are administrated as subcutaneous injections once or twice daily and have a predictable anticoagulant dose-response curve. Therefore, LMWHs are prescribed as a fixed dose based on total body weight and monitoring is generally not required. LMWHs are primarily renally excreted. Thus, patients with renal insufficiency and patients with potentially altered pharmacokinetic profiles (mainly obese and pregnant patients) may be subjected to increased risk of over or under treatment. These patients are usually subjected to TDM by measuring anti-Xa activity.6,7 Whether or not these protocols are entirely evidence-based and should be used is also subject to debate. However, as long as these protocols are recommended, appropriate utilisation is important.
Monitoring anti-Xa levels is a complex clinical challenge. Firstly, since peak anti-Xa levels are reached four hrs after subcutaneous administration of LMWH, sampling for anti-Xa measurement should be organised between 3-5 hrs after LMWH injection. Secondly, a steady state is reached after 2-4 subcutaneous injections. Sampling before steady state concentrations are reached leads to unreliable interpretation of plasma levels and subsequent unjustified dose adjustments, therefore compromising its safety with potentially serious consequences such as thrombotic events or bleeding.8-10
Due to its complexity, we hypothesised that compliance to guidelines concerning anti-Xa monitoring is low. We therefore investigated the indications, timing of sampling, and associated dose adjustments in patients receiving dalteparin (once or twice daily administered LMWH) in the University Medical Centre Utrecht (UMCU).
MATERIALS AND METHODS
Patient population
The study population comprised all patients who were 18 years and older and had their first anti-Xa level drawn between February 23rd and December 30th, 2017. Exclusion criteria were anti-Xa levels drawn for monitoring activity of intravenous heparin or DOAC. Since our hospital uses dalteparin as standard LMWH, the few other LMWHs were excluded.
Study design, data source, and collection
We conducted a single-centre retrospective cohort study at the UMCU, an academic hospital in the Netherlands. Data on the first and repeat anti-Xa level measurements of these patients were collected from the laboratory. Clinical data were abstracted from systematically screened medical records by one researcher (EB). The following baseline characteristics were collected on each patient: age, gender, estimated glomerular filtration rate (eGFR); information about continuous venovenous haemofiltration, haemodialfiltration or haemodialysis; information regarding dalteparin included indication, time of dalteparin administration (as registered in the medical file by the nurse), and dosing frequency; indication for anti-Xa monitoring, anti-Xa level, time of sampling (as registered in the medical file by the laboratory), dose adjustments, and reasons for repeat anti-Xa level measurements. Anti-Xa assays were conducted in our laboratory using Liquid Anti-Xa (Diagnostica Stago, United States of America).
Ethics
The Medical Ethics Committee of the UMCU gave permission to perform this study. This study does not fall under the Medical Research Involving Human Subjects Act.
Recommendations for anti-Xa monitoring
According to the ‘Diagnostics and treatment of venous thromboembolism’ guideline of the UMCU, based on the guideline provided by the Dutch Federation for Nephrology, monitoring anti-Xa activity is indicated when patients receive therapeutic doses of dalteparin and are pregnant, morbidly obese (> 150 kg or Body Mass Index (BMI) > 50 kg/m2), or have renal insufficiency (eGFR ≤ 60 mL/min). Peak levels should preferably be measured four hrs after treatment, but at least within 3-5 hrs after LMWH injection and after at least three subcutaneous injections. According to our guideline, patients on therapeutic dalteparin should receive 200 IE/ kg per day, preferably in one dose. Physicians can decide to give two doses a day, depending on the risk of bleeding. Patients with an eGFR < 30 ml/min or eGFR 30-60 ml/ min start with a normal dose, followed by 50% or 75% of the initial dose, respectively. When dalteparin is used for more than three days, dose adjustment is based on anti-Xa levels. Anti-Xa levels should be 1.0-2.0 U/mL when LMWH is administered once a day and 0.6-1.0 U/mL when administered twice daily.
The guidelines do not recommend anti-Xa monitoring in patients on prophylactic dalteparin, in patients with (suspected) thrombotic or bleeding event, and in patients with a history of renal insufficiency but an eGFR above 60 mL/min at the time of measurement. Additionally, the guidelines do not provide information about the indications for repeat anti-Xa level measurements. As many patients had multiple levels measured, we did examine the reasons for those repeats.11,12
Compliance with recommendations
Medical records were screened for pregnancy, renal insufficiency, and obesity. Anti-Xa level measurements were considered in compliance with the recommendations if the indication was appropriate and if accurate sampling was performed. TDM was considered not in compliance with the recommendations when anti-Xa levels were not sampled 3-5 hrs after subcutaneous injection or if the anti-Xa level was drawn while dalteparin could not have reached a steady state, defined as after less than three injections of dalteparin. Repeat anti-Xa level measurements were classified as appropriate when the previous anti-Xa level was out of range and a dose adjustment was made, when the previous anti-Xa level was incorrectly drawn, when there was a significant change (≥ 10%) in eGFR and the eGFR was below 60 mL/min, or during pregnancy. Incorrect reasons for repetition were thrombotic or bleeding events, restarting LMWH, medical interventions (e.g., surgery), a history of renal insufficiency with eGFR above 60 ml/min at the moment of anti-Xa activity measurement, or otherwise, if the reason for repetition was not clear.
Outcome definitions
The primary outcome was the frequency of non-compliance with the recommendations for anti-Xa monitoring in patients receiving dalteparin. Repeat anti-Xa level measurements were studied to see whether the indication for repetition was appropriate. Secondary outcomes were the consequences for dosage regimens, due to decisions based on these levels.
Statistical analysis
The collected data were entered into a database made with IBM SPSS version 21.0 and frequency analyses were performed. Descriptive statistics were used for demographic data.
RESULTS
Patients
A total of 158 patients with 396 anti-Xa level measurements were included and analysed (figure 1). Baseline characteristics are presented in table 1. This table also shows information regarding patients with an indication for therapeutic dalteparin. Three patients suffered from VTE while using other anticoagulants and were therefore switched to LMWH.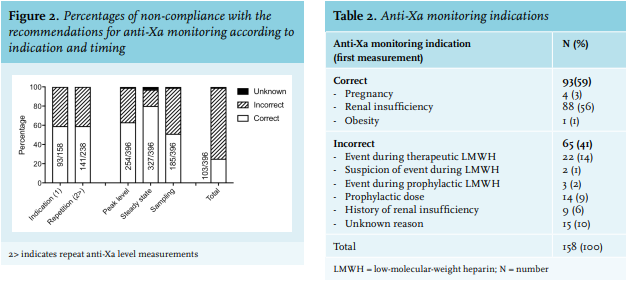
The primary outcome, the frequency of non-compliance with the recommendations for anti-Xa monitoring in patients receiving dalteparin, is illustrated in figure 2. Of all first anti-Xa level measurements, 41% (65/158) had an inappropriate indication (table 2), 36% (142/396) were not drawn as a peak level, and 17% (69/396) were sampled before steady state concentrations were reached. In total, 48% (189/396) were inappropriately sampled, of which, 25% (47/189) were followed by a dose adjustment. Of the repeat anti-Xa level measurements, 41% (97/238) had an incorrect reason for repetition (table 3). Of all initial and repeat anti-Xa level measurements, 74% (293/396) were non-compliant. Of all 396 samples, 118 were followed by a dose adjustment and 64% of these adjustments were based on samples that were either not indicated or performed at the wrong time. In total, 245 anti-Xa samples were below the target level. No dose adjustment was made in 129/245 patients, the dose was increased in only 98/245 patients, and three anti-Xa level measurements were followed by a dose reduction. Considering a (suspected) event during therapeutic LMWH as an appropriate indication or reason for repetition, although not stated in the guidelines, 71% (279/396) of all measurements were non-compliant.
DISCUSSION
Non-compliance with recommendations for anti-Xa monitoring in patients receiving dalteparin was high in our academic hospital during 2017. To our knowledge, this is the first study reporting on compliance with recommendations for anti-Xa monitoring in patients receiving dalteparin. Because treatment and TDM protocols are relatively complicated, it is highly likely that these findings are not unique to our hospital. In fact, multiple studies looking at TDM compliance of other anticoagulants show very similar results. All of these retrospective, single centre studies were also conducted in large first world country hospitals.13-16
Kufel et al. determined the frequency of correctly drawn anti-Xa levels in patients treated with enoxaparin in accordance with predefined criteria and reported the number of dose adjustments based on incorrectly drawn anti-Xa levels. They included 59 patients with 74 anti-Xa concentrations and concluded that 77% of the anti-Xa levels were incorrectly drawn and often resulted in repeat anti-Xa level sampling; 42% of dose adjustments were based on incorrectly drawn anti-Xa levels.13 Dekker et al. investigated the compliance of prescribers with local guidelines for monitoring of enoxaparin in 67 patients and found that 38 patients (57%) were not appropriately monitored.14 Sacha et al. showed that, in patients treated with enoxaparin, only 44/76 (58%) of the LMWH anti-Xa levels were drawn as a peak level as recommended by the CHEST guidelines.15
Taken together, these three studies also indicate that the majority of anti-Xa measurements is either not indicated or collected at the wrong time, which resulted in unjustified dose adjustments. In our centre, patients on a once daily dose regimen receive dalteparin at 18.00 hrs and patients on a twice daily dose regime receive dalteparin at 08.00 hrs and 18.00 hrs. Blood is therefore drawn at 12.00 hrs and 20.00 hrs. This is a burden for the nurses in evening shifts and for the lab, as these samples arrive late in the evening. Compliance with protocols requires feasibility to implement its practical aspects within the work routine of the nurses and the lab. This again shows us that protocols that are practically difficult to adhere to are prone to be incorrectly followed. Even in the setting of our centre (an academic hospital), compliance is low, informing us that we either need to drastically reconsider our protocols or think of alternative solutions.
The studies mentioned above strengthen our results and support a broader need for anti-Xa protocol evaluation and adjustment in hospitals around the globe.
The main strength of this study is the completeness of “real world” data obtained through the laboratory reports with all anti-Xa levels. Our study also describes the largest number of patients compared to all other studies on anti-Xa level monitoring and is the first to describe monitoring in patients treated with dalteparin.
There are some limitations, however, that need to be addressed. First, this study had a retrospective design. This could lead to potential inclusion bias. For example, the number of patients that were not monitored but should have been monitored is unknown. Dekker et al. showed that 18 of the 67 patients (27%) who received enoxaparin had an indication for monitoring according to their local guideline but were not monitored.14 In addition, patients with renal failure possibly need routine dose adjustments based on their eGFR. Kikkert et al. assessed Dutch antithrombotic treatment strategies for acute coronary syndrome in light of the European Society of Cardiology guidelines and showed that dose adjustments of LMWH therapy for patients with renal insufficiency were not applied in 71% of the hospitals.17 Compliance to this specific part of the protocol is also important in LMWH dosage regimens, but this study focused on TDM and therefore only investigated whether anti-Xa monitoring and eventually dose adjustments based on anti-Xa levels were performed correctly. Finally, we used time of registry to check if peak levels were monitored, but this registry time does not always reflect the real time of last administration.
Second, the criteria that identify events as non-compliant could be debated. Indications for anti-Xa monitoring are not always registered by treating physicians, which in our study was classified as non-compliant. However, it is possible that some of these events were indicated correctly, and therefore influenced the results.
Third, we identified off-protocol indications that are not necessarily wrong. For example, several patients were sampled to check compliance to therapy when they were admitted to the hospital with a VTE or recurrent VTE. In this study, we categorised these anti-Xa sampling indications as incorrect, which can be debated. However, when we further analysed our data, we found that 71% of the anti-Xa measurements could still be classified as non-compliant, even when an event or suspected event is considered an appropriate indication or reason for anti-Xa assay repetition.
Another point of discussion are the samples drawn prior to reaching steady state concentrations with anti-Xa activity above the upper limit. At this moment, a dose adjustment can already be made. However, correct dose adjustments by clinicians can only be made when they are aware of the exact times of dalteparin administration and blood sampling, which in practice is usually not the case. In our cohort, of the 69 patients who were sampled before steady state concentrations were reached, only four patients had an anti-Xa activity above the upper limit. None of them received a dose adjustment.
Another category of questionable compliance is formed by patients with a fluctuating eGFR. When anti-Xa levels were measured while the eGFR was above 60 mL/min, this measurement was classified as incorrect, while the rationale to sample anti-Xa in patients with fluctuating eGFR is defendable.
In summary, this study showed that non-compliance with recommendations for anti-Xa monitoring is high, resulting in unjustified dose adjustments. Although TDM is straightforward in principle, in practice it is a complex clinical process, and it is highly likely that the findings in this study are universal.
To improve large scale anti-Xa monitoring, we recommend that hospitals reconsider administration and blood collection times in order to facilitate anti-Xa monitoring within reasonable working hours, as this will relieve the burden on shift workers. Education and automatic alerts will help create awareness and thereby probably increase compliance. Finally, the outcome of anti-Xa monitoring on clinical endpoints is questionable. We therefore think that this practice as a whole should be reconsidered.
In conclusion, we strongly recommend revisiting in-hospital LMWH drug monitoring protocols and if applicable, evaluate compliance and awareness.
DISCLOSURES
All authors declare no conflicts of interest. No funding or financial support was received.
REFERENCES