

KEYWORDS
Flucloxacillin, serum concentration, absorption, Staphylococcus aureus
INTRODUCTION
Despite advances in antibacterial therapy and stewardship, the effective treatment of severe Staphylococcus aureus infections remains an important clinical challenge. Globally, the incidence of severe staphylococcal infections remains high,1-2 which is partly due to the increasing use of indwelling catheters, vascular and orthopaedic prostheses, and prosthetic heart valves.3 Severe S. aureus infections are associated with a high mortality rate and with associated complications (including infective endocarditis) being more prevalent compared to other bacterial infections.4 It is clear that the effective management and treatment of severe S. aureus infections is essential. The treatment of choice for severe S. aureus infections depends on concomitant variables including pathogen antibiotic susceptibility, patient factors (including underlying co-morbidities and concurrent medication), and physician preference. In countries with low endemic methicillin-resistant S. aureus (MRSA) rates, such as the Netherlands,5,6 intravenous (IV) flucloxacillin is the preferred choice of treatment because of its bactericidal activity and narrow-spectrum of activity. Continuous IV infusion of flucloxacillin is followed by a course of oral flucloxacillin, which allows for earlier discharge of the patient from the clinic and has a reduced risk of catheter-associated complications. Flucloxacillin is rapidly absorbed with maximal serum concentrations observed at approximately one hour after intake. It has a high degree of protein binding (approximately 90%) and an elimination half-life of one hour.7 Of note, previous studies have demonstrated a high degree of variability of flucloxacillin absorption following oral administration,7-9 and the mechanisms underlying the observed variability remain unclear. It is of clinical importance to ensure that oral flucloxacillin is adequately absorbed in patients and that therapeutic serum levels are maintained, in favour of effective treatment of the underlying bacterial infection. Knowledge of the full pharmacokinetic profile of flucloxacillin in serum and the minimum inhibitory concentration (MIC) of the isolate are indicators of adequate oral dosing. The breakpoint MIC for flucloxacillin-susceptible S. aureus, defined as the highest MIC value indicating susceptibility, is commonly defined as < 0.5 mg/l of free drug. Given its high binding capacity, this will translate into a total serum drug concentration of 5 mg/l. Therefore, we routinely accept serum flucloxacillin concentrations of at least 10 mg/l as therapeutic levels, as these are associated with protein-free drug concentrations of > 1 mg/l, which is well above the MIC.10,11 This level of exposure was therefore commonly accepted and associated with the efficacious treatment of susceptible S. aureus strains by beta-lactam antibiotics.11 The oral absorption test (OAT) is used to ensure an efficacious switch from intravenous to oral therapy and has been routinely performed at our institution (Leiden University Medical Centre, the Netherlands). Results have shown that in approximately 10% of patients, the absorption of oral flucloxacillin was insufficient to reach therapeutic levels (i.e., a maximal serum concentration increase of < 10 mg/l).9 The OAT format was laborious and error-sensitive, as it required the cessation of the continuous IV flucloxacillin administration eight hours prior to oral intake of the test dose. Recently, we demonstrated that a simplified version of the OAT performed similarly to the classical OAT, was easy to administer and could be implemented in hospital pharmacies at low equipment and staff costs.9 However, our key concern was the small patient sample (43 patients) and retrospective design of that study. In the current study, we aimed to confirm our previous findings in a larger patient population, to investigate whether a simplified OAT can be utilized in a clinical setting to guide antibiotic treatment in patients with severe S. aureus infections, and to screen for factors associated with the previously observed inter-individual variability in oral flucloxacillin absorption.
PATIENTS AND METHODS
This study complied with institutional guidelines and Dutch law, as the evaluation concerned daily routine practice that adheres to the law on the medical treatment agreement (WGBO; Wet op de Geneeskundige Behandelings Overeenkomst). Hence, separate medical ethical approval was not needed.
Patients
The evaluation period included adult patients admitted between 2011 and 2017 to Leiden University Medical Centre, Leiden, the Netherlands. Data were retrospectively collected from 196 hospitalised patients receiving continuous IV flucloxacillin and scheduled for oral flucloxacillin treatment. No potentially eligible patients were excluded from the evaluation.
Flucloxacillin Oral Absorption Tests 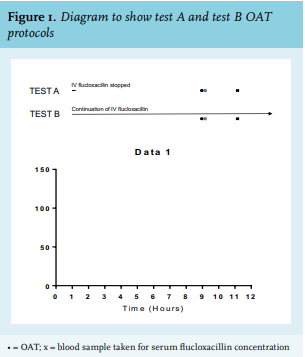
We evaluated two separate test protocols to assess the oral absorption of flucloxacillin (figure 1). The simplified version of OAT (test B) was already implemented. The classical OAT (test A) was occasionally performed due to ingrained habits. Test A commenced with an overnight fast and interruption of continuous IV infusion of flucloxacillin for eight hours. Thereafter, an oral test dose of flucloxacillin (1 g) was given. Blood samples for serum flucloxacillin concentrations were taken at baseline, and at one hour after oral dosing, according to the expected Cmax of flucloxacillin. Because of the expected inter-patient variability in Cmax levels, it was decided to add a measurement at two hours. This also allowed for a better estimation of flucloxacillin absorption in case of diminished gastrointestinal motility. Test B required the continuation of IV infusion of flucloxacillin. An oral test dose of flucloxacillin (1 g) was given after a one-hour fast. Measurement of serum flucloxacillin concentrations were performed at similar time-points as in test A (figure 1). Adequate absorption was defined as an increase of serum flucloxacillin concentration of at least 10 mg/l from baseline at either sampling time. Because of the high level of protein binding and renal excretion of flucloxacillin, albumin and serum creatinine were also assessed in patients prior to oral flucloxacillin dosing.
Flucloxacillin assay
Flucloxacillin serum concentrations were determined using a validated high-performance liquid chromatography (HPLC) method with ultraviolet detection (all equipment from Dionex Corporation, Sunnyvale, CA, USA). We added 10 µl of a 1 mg/l of cloxacillin solution (Sigma) and 0.5 ml of acetonitrile (Promochem) to 0.5 ml of a thawed patient serum sample. The samples were then vortexed and subsequently centrifuged for five minutes at 25,000 g. Thereafter, 0.8 ml of the supernatant was transferred to a 10 ml polypropylene test tube, and 3.5 ml of chloroform (Merck) was added. The samples were vortexed and centrifuged for three minutes at 5,500 g. We mixed 0.1 ml of the aqueous upper layer with 0.1 ml of acetate buffer (0.1 mole/l), and 20 ml of this solution was assayed by HPLC.
The chromatographic system consisted of an octadecylsilica Hypersil stationary phase (3 mm particle size, length 12.5 cm, id 4.6 mm), and a mixture of 1 mole/l acetate buffer solution (pH 6), water, and acetonitrile (40 + 710 + 250, vol/vol) as mobile phase. Flow rate was 1.0 ml/ min, and detection took place at a wavelength of 210 nm. A flucloxacillin reference solution in serum was pre-treated using similar methodology as in the patient samples. This solution was used to determine the flucloxacillin/ cloxacillin signal ratio in patient samples. From this ratio, serum concentrations of flucloxacillin were calculated. The lower limit of quantification was 3 mg/l, and the assay showed linearity for flucloxacillin concentrations up to at least 100 mg/l. For a quality control sample of a predefined concentration (40 mg/l) we found a mean concentration of 44.4 mg/l (111%) after 15 tests over a one-month period, with a coefficient of variation of 4.0%.
Data analysis
Demographics of the study population were summarised. Baseline flucloxacillin levels, age, serum creatinine concentration and serum albumin concentration in relation with the maximal increase of flucloxacillin concentrations were visually explored. The change in maximal increase of flucloxacillin levels between test A and test B, and between males and females was tested using an unpaired Student’s t-test. A p of 0.05 was considered statistically significant.
RESULTS
Of the 196 patients (85 females and 111 males) who were treated with IV flucloxacillin, the individualised dose of continuous infusion ranged from 6-12 g/d. Baseline characteristics of patients were comparable between OATs A and B (table 1). Two measurements at two hours after dosing were removed from the analysis due to unrealistic outliers (> 200 mg/l), probably because samples were taken erroneously from the flucloxacillin catheter. There was a difference in maximal increase of flucloxacillin absorption from baseline (figure 2). The median (IQR) maximal increase was 22.0 (15-31.25) mg/l for test A and 21.5 (13-32.25) mg/l for test B (figure 2). There was no significant difference in maximal increase of serum flucloxacillin levels between tests A and B (p = 0.744). No relationship could be identified between any of the covariates and the maximal increase of serum flucloxacillin concentrations (figure 3A-D). The inter-subject variation in flucloxacillin seemed to increase with increasing age. No statistical tests were performed due to insufficient data. In 26 (13.27%) of the 196 patients, the maximal increase of flucloxacillin concentration did not reach the predefined target of 10 mg/l. This was found in 10.7% patients using test A and 13.7% patients using test B.
There was no significant difference in maximal increase of serum flucloxacillin concentration between male and female subjects for test A (p = 0.80) and test B (p = 0.95). Additionally, there was no relationship between both serum creatinine and serum albumin levels and the observed maximal increase of serum flucloxacillin concentration (figure 2). Serum creatinine (n = 143) and albumin (n = 48) samples were not available for all included subjects (figure 3, C and D).
Most of the maximal flucloxacillin concentrations were reached at two hours after dosing (54.6%). In tests A and B, 45.4% of the apparent maximum concentrations were achieved one hour post dose and 54.6%, two hours post dose. 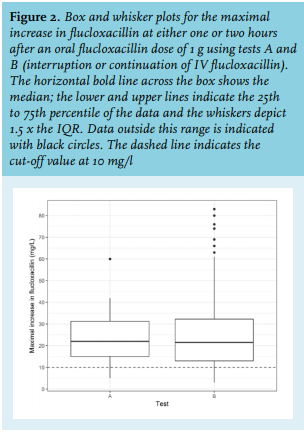
In the current study, we confirmed the finding of our previous study, wherein we demonstrated that a simplified version of the OAT was easy to perform and could be implemented in hospital pharmacies at low equipment or staff costs, and performed similarly to the classical OAT.9 In the current study, we observed that there was no significant difference in maximal increase in serum flucloxacillin levels from baseline between tests A and B, the classical and simplified version of the OAT. As flucloxacillin has a wide therapeutic window, it was deemed that the short-term exposure to higher levels of flucloxacillin in the simplified OAT was safe. As such, the simplified OAT is not only a safe, viable alternative, but also has practical advantages compared to the classical OAT; for example, nursing staff is no longer required to stop and start IV pumps, which saves approximately 20 minutes of nursing staff time per test.
A large inter-subject variability in oral flucloxacillin absorption was observed in our patient sample. It should be noted that outliers were seen in the simplified OAT group, which adds an extra level of variability in absorption. We believe that this is mainly caused by the larger sample size in the simplified OAT group. Median and IQR values for both tests were comparable. Our study highlighted that a significant proportion of all patients (13%) demonstrate insufficient drug absorption of flucloxacillin. This finding is in agreement with the results of our previous study,9 and of previously published pharmacokinetic data.7,8,10 Our results further showed that there was no correlation between serum creatinine and serum albumin levels and the observed maximal increase of serum flucloxacillin concentration. In tests A and B, 45.4% of the apparent maximum concentrations were achieved one hour post dose and 54.6%, two hours post dose. This suggests that the Cmax likely occurred sometime in between the sampling times. Future research may focus on optimising the sampling times to identify sufficient absorption to reduce the required samples from two to one. However, as covariates and high variability may impact the absorption process,12 two samples are recommended to perform the absorption test at this stage. Since Cmax mainly depends on the extent and rate of drug absorption, minor or no effects could be expected from renal function or serum albumin. Knowing that flucloxacillin is predominantly excreted by the kidneys and is associated with a high degree of protein binding (approximately 90%), the observed lack of correlation suggests that both renal function and variation in serum albumin are not responsible for the observed inter-individual variability in oral flucloxacillin absorption in our patient sample.
The results of both simplified and classical OAT show that 45.4% of the maximum flucloxacillin concentrations were achieved one hour after administration and 54.6%, two hours after administration. This confirms the expected inter-patient variability in Cmax levels, and justifies the use of two sampling time points in our study.
It should be noted that a substantial proportion of patients (14%) in the simplified and classical OAT groups did not absorb well. This could be explained by genetic variation in drug transporter enzymes or enzymes involved in the first-pass metabolism of flucloxacillin; however, the mechanism underlying the observed inter-individual variability in oral flucloxacillin absorption remains undetermined. Based on our personal experience and expertise, we believe that patients with an adequate level of oral absorption will generally demonstrate consistent levels of absorption in repeated tests. On the other hand, patients who are identified as poor absorbers, generally display variable levels of absorption during retesting. The reason for this variability remains unclear. Factors such as gastric emptying and intestinal motility could play a role; for example, late absorption of rifampin was associated with delayed gastric emptying such as diabetes mellitus.13 Polymorphisms in the gut could also result in a reduced absorption of flucloxacillin and drug transporter polymorphisms like P-glycoprotein, or first-pass enzymes could further complicate the pharmacokinetic profile of variable flucloxacillin absorption. Another explanation could be a pharmacogenomic mechanism, since a substantial proportion of the patient sample in our study showed levels of inadequate absorption. Currently, there is no evidence for the involvement of genetic variability leading to a differentiated expression or function of metabolic enzymes such as cytochrome P450 (CYP) or drug transporters, while un-identified polymorphisms in CYP gene expression and/or enzyme activity could indeed play a role in the increased hepatic breakdown of flucloxacillin. Similarly, genetic variation in P-glycoprotein polymorphisms or hepatic enzymes may influence the absorption and first-pass effect of flucloxacillin, resulting in inter-individual differences in flucloxacillin absorption and, hence, peak levels. We do know that a high dose of IV flucloxacillin for a minimum of two weeks prior to oral dosing might be responsible for the induction of increases in gene expression of drug transporters and/or CYP enzymes, as previous studies have reported the induction of hepatic CYP 3A4 and P-glycoprotein by flucloxacillin.14-16 Therefore, there is a need for novel studies exploring how pharmacogenomics affect the pharmacokinetic profile of flucloxacillin.
Limitations of our study were the retrospective design and the absence of clinical assessments.
In summary, we have designed and confirmed that a simplified OAT can be utilized in a clinical setting to guide antibiotic treatment in patients with severe S. aureus infections. The HPLC/ultraviolet flucloxacillin assay can be easily performed by most labs, and can be implemented in hospital pharmacies with limited equipment and staff costs. We have demonstrated that this adapted OAT can be safe, less expensive, less time consuming, and less error-sensitive, compared to the classical OAT, and can adequately identify patients with insufficient oral flucloxacillin absorption without interruption of IV therapy; this is advantageous since serum levels will not drop below therapeutic levels. As the mechanism(s) underlying the observed inter-individual variability in oral flucloxacillin absorption remain elusive, it is vital to develop a quantitative test to clinically assess the efficacy of oral absorption of flucloxacillin to ensure patient safety and the efficacious treatment of underlying bacterial infections. For optimal management of patients with severe S. aureus infections, we strongly encourage fellow clinicians to adopt and implement our simplified OAT in clinical practice.
DISCLOSURES
All authors declare no conflicts of interest. No funding or financial support was received.
ACKNOWLEDGEMENTS
We thank Tessa Nelemans (Master’s student, Biomedical Sciences, Leiden University Medical Centre, the Netherlands) for support in data analysis.
REFERENCES