

KEYWORDS
Bone marrow, FDG-PET CT scan, haemato-oncological diseases, inflammation
INTRODUCTION
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) combined with computed tomography (CT) (18F-FDG PET/CT) is an established imaging modality in the diagnosis, prognosis, and evaluation of patients with various haematological conditions, based on uptake of its tracer 18F-FDG in cells with high glycolytic rates.1,2
However, haematopoietic cells in the bone marrow compartment have constitutively considerable rates of glycolysis, which also results in 18F-FDG uptake on PET/ CT scans. Given this physiological background signal, it is of importance to recognize specific patterns of FDG uptake in the bone marrow in order to accurately interpret 18F-FDG PET/CT scan findings.
This review aims to give a comprehensive overview of the different patterns of 18F-FDG uptake in PET/CT scans in common haematological conditions, accompanied by illustrative cases.
BASIC PRINCIPLES OF FDG-PET/CT
The most commonly used tracer is a glucose analogue, fluorodeoxyglucose (FDG), labelled with the radionuclide 18F, with a half-life of 109 minutes. 18F-FDG is taken up by metabolically active cells and subsequently phosphorylated. Because of this, it is unable to enter the citric acid cycle and thus accumulates intracellularly. Most cells rely on glucose as their primary energy source under steady state conditions, and cells with high energy demand increased rates of glycolysis, in both the presence and absence of available oxygen. Based on this principle and due to often significant 18F-FDG uptake in tumour cells, 18F-FDG-PET/CT is a sensitive technique to detect and stage oncological diseases and assess treatment responses.3-6 However, other factors influencing the metabolic rate of the haematopoietic system, for example inflammation or increased haematopoiesis, should be discriminated. The basic principles of PET and CT are further explained by figure 1.
Physiological FDG uptake in bone marrow
About 4% of total body weight is bone marrow tissue. Depending on age, adult bone marrow produces between 1011-1012 new haematopoietic cells per day, thereby maintaining the number of circulating blood cells.7 In addition, the haematopoietic system is capable of responding quickly by increasing haematopoietic activity on demand, for example, during severe infection or repopulation after myeloablative chemotherapy. 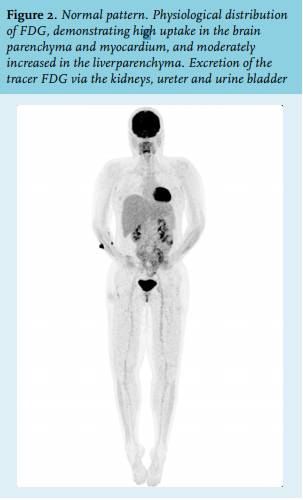
By considering four arguments, the differential diagnosis of abnormal 18F-FDG uptake patterns can be narrowed down to a reasonable working diagnosis:
Level of 18F-FDG uptake (SUVmax): normal (SUVmax ≤ 3); moderately increased above liver FDG values (SUVmax ≥ 4, ≤ 10); extensively increased (SUVmax ≥ 11).
Distribution of 18F-FDG uptake: central compartment versus peripheral epiphysis, involvement of the spleen, lymph nodes and/or organs, and diffuse versus focal.
Coinciding changes of the bone structure as observed by CT, which result from osteoblasts/osteoclasts activity and bone marrow cellularity.
Clinical context, including medical history and laboratory findings.
In the following paragraphs, different benign and malignant haematological conditions supported by illustrative cases are described using these four arguments. Table 1 provides an overview of 18FDG PET-CT scan findings for each haemato-oncological condition.
Benign conditions of increased FDG-update in bone marrow
Haematopoietic activity can be physiologically extensively increased in certain clinical conditions such as systemic inflammatory response to infection, chronic inflammation, or growth factor administration following myelotoxic chemotherapy. The central mechanism is the increased presence of growth factors, which leads to a higher affinity of glucose transporters for deoxyglucose.8,11,13 Here, the pattern of FDG uptake of the central compartment resembles normal haematopoiesis, for example, diffuse and homogeneous, yet demonstrates an increased intensity (figure 3). Under certain circumstances, the spleen may also be involved, reflecting splenic extramedullary haematopoiesis.11,14,15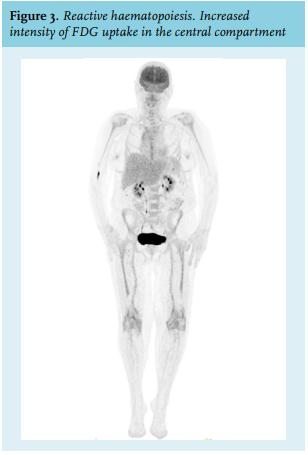
Inflammation defines an immunological process caused by tissue damage and/or infection. Inflammation leads to an increased number of glucose transporters within the affected tissue, while infiltrated granulocytes and macrophages also use glucose as their energy source.16 Both events result in a homogeneous increased FDG uptake in the bone marrow and lymph nodes with a possibly moderate SUV.8,17 Inflammation due to infection induces systemic effects resulting in a diffuse FDG uptake in the bone marrow and spleen. The latter must not be misinterpreted as a splenic infection or splenic tumour which appears as focal lesions.8,9,15 To avoid misinterpretation, it is important to correlate the patient’s clinical presentation including inflammatory parameters with the FDG-PET/CT-scan.
Growth factors
Granulocyte-colony stimulating factor (G-CSF) may be used to accelerate granulocytic recovery following myelotoxic chemotherapy or compensate for disease-related impairment of granulopoiesis. A moderate increase in FDG uptake can be observed in the bone marrow within these patients.9,11
Elevated, diffuse FDG uptake due to increased haematopoiesis may be misinterpreted for a bone marrow malignancy or disorder. Thus, information regarding medical history and laboratory findings are important to establish a correct diagnosis.18 Discontinuation of G-CSF treatment leads to a rapid decrease and normalisation of FDG uptake in the bone marrow.11
As elevated FDG uptake can be demonstrated as soon as three days after the start of treatment with G-CSF and normally resides after three days post-treatment completion,11,19 it is advised to perform FDG-PET imaging with a delay of at least five days after discontinuing G-CSF. To summarize, increase of haematopoiesis due to an either endogenous or exogenous inflammatory stimulus induces a specific pattern of FDG accumulation characterised by a homogenous and highly intensive FDG uptake observed at the physiological locations.
Clonal diseases of increased FDG uptake in bone marrow
Erdheim Chester (EC), Langerhans Cell Hystiocytosis (LCH), and Haemophagocytic lymphocistiocytosis (HLH) are rare disorders within the histiocytic cell lineage. EC is a non-Langerhans cell, multisystemic granulomatosis, characterised by accumulated foamy histiocytes present in the long bones and large blood vessels. In some cases, the heart and central nervous system are involved. Recently, several mutations have been identified supporting the clonal nature of the disease and its pro-inflammatory cytokine phenotype. In 50% of all cases, patients present themselves with focal bone pain and are mostly in their adult age. Focal moderately-active lesions in the bone marrow are seen on the FDG-PET/CT scan which may also be visualized by bone scintigraphy (figure 4).20-23 Typical bone involvement is bilateral with symmetrical cortical thickening.24 Osteosclerosis is seen in the long bones, except for epiphyses, the axial skeleton, and mandible. In addition to osseous manifestations, histiocytes often accumulate in different tissues leading to involvement of the kidneys, heart, lungs, and brain. Due to the rarity of this disease, it can be easily overlooked or mistaken for a primary bone malignancyFDG is not a cancer-specific agent, and knowledge of the differential diagnosis of benign FDG-avid bone alterations that may resemble malignancy is important for correct patient management, including the avoidance of unnecessary additional invasive tests such as bone biopsy. This review summarizes and illustrates the spectrum of benign bone conditions that may be FDG-avid and mimic malignancy, including osteomyelitis, bone lesions due to benign systemic diseases (Brown tumour, Erdheim-Chester disease, Gaucher disease, gout and other types of arthritis, Langerhans cell histiocytosis, and sarcoidosis. Histological biopsy is needed to confirm diagnosis. PET-CT scans are useful for initial assessment and follow-up of lesions, including pituitary involvement.14,24-26
LCH is another rare disease of abnormal clonal proliferation of myeloid dendritic cells, defined as Langerhans cells. This disease is usually diagnosed during childhood. Recently, it was discovered that BRAFV600E mutations characterise the disease in the majority of patients.27-29 These cells are able to infiltrate tissue, preferably bone tissue such as flat bones like the skull, pelvis, and ribs, resulting in osteolysis. FDG uptake is asymmetric with a moderate SUVmax, depending on the phase and metabolically-active sites of disease.30 Osteolysis is absent in EC. During early stages, LCH can be mistaken for malignant bone tumours. Similar to EC, histopathologic examination of one of the infiltrated tissues is needed to confirm diagnosis.14 In general, EC and LCH may be difficult to discriminate in clinical practice due to considerable overlap in clinical presentation, imaging findings, and laboratory findings.
Finally, HLH is a non-malignant syndrome and characterised by overproduction of cytokines resulting in activation of cytotoxic T cells, natural killer (NK) cells, and macrophages, ultimately culminating in uncontrolled hyperinflammation. The excessive immune activation results in the clinical hallmarks of the disease, i.e. fever, hepatosplenomegaly, cytopaenias and hyperferritinaemia. Hereditary or primary HLH often manifests itself during infancy or childhood due to a variety of underlying heterogenous genetic mutations. Acquired or secondary HLH may result from a strong immune activation in response to infection (in particular Epstein Barr Virus), malignancy, or an autoimmune disorder.31 On images, PET-CT demonstrates a diffuse FDG uptake pattern in the spleen and a normal uptake in the bone marrow (figure 5).32-35 Other organs may be affected as well.
Malignant conditions of increased FDG uptake in bone marrow
Primary myelofibrosis
Myelofibrosis is a clonal disorder involving a multipotent haematopoietic stem cell. This disease is characterised by excessive, though often ineffective, proliferation associated with pro-inflammatory cytokines, bone marrow fibrosis, and extramedullary haematopoiesis. Constitutional symptoms, cytopaenia due to bone marrow failure or transformation to acute myeloid leukaemia, symptomatic hepatosplenomegaly, and thromboembolic events may complicate its clinical course.
In over 50% of patients, a typical mutation in the JAK2 gene (V617F mutation) can be identified, which results in an alleviation of the protein’s physiological inhibitory status, thereby facilitating the erythropoietin and thrombopoietin receptors to become autonomous with respect to growth factor. Currently, the JAK1 and JAK2 inhibitor ruxolitinib and interferon are non-curative, but are effective therapies, suppressing inflammatory cytokine production and thereby reducing associated clinical symptoms as well as haematopoietic progenitor proliferation. Allogeneic stem cell transplantation remains the only potential curative treatment for now.36
With PET-CT, the bone marrow shows a diffuse, homogeneous pattern of FDG uptake (figure 6). Due to extramedullary haematopoiesis, an increased uptake may also be seen in the spleen, liver, and central skeleton. Spleen involvement seems to be positively correlated to bone marrow disease stage and represents the degree of extramedullary haematopoiesis. Intensity in bone marrow uptake decreases over time due to fibrosis and osteosclerosis, resulting in hypocellular and fibrotic parts lacking FDG uptake with narrowing normal haematopoiesis to only distal compartments and an increased FDG uptake in the spleen.16,37,38
Multiple myeloma
Multiple myeloma (MM) originates from neoplastic plasma cells, which often accumulate in the bone marrow, cortical bones, and sometimes the extraosseous localisations. This disease often presents with spontaneous bone fractures, hypercalcaemia, renal insufficiency, and anaemia.39 Bone marrow biopsy will show an excessive amount of plasma cells.6,40 FDG-PET/CT scans may show focal osteolytic bone lesions and diffuse osteopenia with a moderate FDG uptake (figure 7). Higher and mixed FDG uptake are associated with fast progression and poor prognosis. PET-CT has a central role in both visualising extramedullary plasmacytomas as well as evaluating treatment response in non-secreting myeloma.
Acute leukaemia
Acute leukaemias originate from a clonal proliferation of malignantly transformed myeloid or lymphocytic progenitor cells, ultimately replacing normal haematopoiesis and inducing progressive and severe bone marrow failure. Due to pancytopaenia, patients are susceptible to infection, bleeding, and suffer from anaemic symptoms.
FDG-PET may be used when extramedullary disease is suspected. Due to the diffusely infiltrating hallmark of the disease, FDG-PET/CT demonstrates a typical diffuse pattern in which, depending on the proliferating rate of the disease, an extensive SUVmax can be found (figure 8).41 Under extensive disease conditions, the residual normal haematopoiesis may have shifted to peripheral sites, e.g., the tibia, resulting in hypercellular bone marrow that is visible as hyperdense bone marrow by CT scan. In addition to bone marrow localisation and depending on the specific leukaemic subtype, leukaemic cells may be present in extramedullary organs, e.g. in the liver, spleen, lymph nodes, and skin.41,42 Recently, we described a case of recurrent acute myeloid leukaemia with pericardial and abdominal myeloid sarcomas lacking bone marrow involvement.43
Mature lymphoma
Both for Hodgkin lymphomas (HL) and non-Hodgkin lymphomas (NHL), the attributable role of FDG-PET/ CT has been extensively increased.44-49 HL and NHL are neoplasms derived from mature lymphoid B or T cells. The stage of differentiation of these malignant immune cells determines the behaviour of the neoplasm.44
Staging of lymphoma is based on the Ann Harbor classification and involves the sites involved (nodal, extranodal) and disease distribution. FDG-PET/CT scans are routinely used in staging and assessment of treatment outcomes in Hodgkin and high-grade NHLs such as diffuse large B-cell lymphoma and Burkitt lymphoma, as disease can be typically visualised by high SUV levels.45 For diseases with lower rates of metabolic activity such as follicular lymphoma, this modality is less suitable due to the difficulty in interpreting normal to only slightly increased SUVmax levels. Consequently, FDG-PET/CT is not advised to be used in intermediate and low-grade NHLs.49-52
Both HL and high-grade NHL may show focal abnormalities on the FDG-PET/CT image (figure 9). Yet, due to a (respectively) lymphatic versus haematogenous spreading, HL of stage II and higher demonstrate FDG avidity in contiguously linked lymph nodes. In contrast, NHL is characterised by skipped lesions with FDG avidity in different lymph nodes throughout the body, together with involvement of different organs and bone marrow.42 Finally, as definite diagnosis of high-grade lymphomas requires histopathologic examination, PET-CT may support this by visualising the most intense and thereby preferred location of biopsy.41,45
CONCLUSION
PET-CT has increasingly been implemented in the diagnostic process of haemato-oncological conditions. In this review, we described patterns of 18F-FDG uptake in the bone marrow in benign, inflammatory, and haematooncologic conditions, and provided arguments that may aid in the accurate interpretation of 18F-FDG PET-CT in clinical practice.
DISCLOSURES
All authors declare no conflicts of interest. No funding or financial support was received.
REFERENCES