

Dear Editor,
Despite extensive screening measures to prohibit transfusion-transmissible infections, blood transfusion is not, and may never be, 100% safe. This is because new and re-emerging viruses are spreading more widely and rapidly and may pose a yet undefined risk to transfusion safety.1,2 In order to be transmittable through blood, there should either be a period of asymptomatic viraemia, or the infection should be subclinical in a proportion of the infected population.3,4
Hepatitis E virus (HEV) is a member of the family Hepeviridae and predominantly transmitted by the faecal-oral route through contaminated water and food. However, HEV can also be transmitted through donation of HEV-infected blood.3-6 Worldwide, HEV is not included in the standard screening programs in blood banks.
Patients usually recover spontaneously, although infection with HEV can be more severe in pregnant women and immune-compromised patients, especially those undergoing organ transplantation.3-7 HEV is classified into four genotypes (1-4).3,4 Genotype 3 is the main cause of human infections in the United States and Europe and can be transmitted to humans through ingestion of raw meat from infected animals, mostly swine.8,9,10
The global incidence of hepatitis E infection has increased, and is an important public health concern in developing countries.1,3,4,8
Several international studies have described the presence of HEV antibodies and HEV-RNA in the serum of healthy blood donors1,6,10-12 with seroprevalences ranging between 2.3-32.6%.1,8,10 Latin America, which is closely related to the South Caribbean, is considered to be an endemic region with seroprevalences between 1.5-20%.8 In the Netherlands, Slot et al. showed that 27% of the donations tested positive for anti-HEV IgG, of which 3.5% also tested positive for HEV IgM; four of them were viraemic.1 Based on their findings, the risk of HEV transmission from blood donors to blood recipients through blood transfusion is calculated to be around one per day.
The extent to which HEV circulates in Curaçao and Aruba in the South Caribbean is as yet unknown. The goal of this study was therefore to determine the HEV seroprevalence and potential risk of transmission via blood donors in the South Caribbean area.
Study setting
Curaçao and Aruba are two Caribbean islands, 40 miles north of the coast of Venezuela, with an estimated population of 150,000 and 107,000 people respectively. Donated blood from Aruba is stored in the blood bank in Curaçao. Both blood banks are certified yearly by Sanquin, which is the European Blood Bank Certification Organisation that works according to ISO standards.13
Data collection
A cross-sectional serosurvey was conducted in April and May 2011 in blood plasma of all blood donors. Informed consent was obtained at the moment of donation.
Blood donations of both islands, around 10,000 per year, are routinely screened for human immunodeficiency virus, hepatitis B and C virus, human T-lymphotropic virus 1 and 2 and syphilis. Afterwards, samples are stored for two years at -80°C. Persons belonging to pre-defined risk groups for the transmission of viral diseases (e.g., men who have sex with men, people who have had a febrile episode < 6 months before donation) are not allowed to donate blood.
Diagnostics
A total of 1 ml blood plasma was collected from each donor. Samples were serologically analysed for anti-HEV IgG and anti-HEV IgM, with subsequent testing for HEV-RNA at the department of ViroScience of the Erasmus Medical Centre in Rotterdam. Sera were tested using the hepatitis E IgG-ELISA WE-7296 and hepatitis E IgM-ELISA WE-7196 (both Wantai Diagnostics, Beijing, China). Positive IgG-HEV samples were screened for the presence of HEV RNA by an internally controlled quantitative real-time polymerase chain reaction with primers detecting all four genotypes and validated according to International Standards Organisation guidelines 9001 and 15189.
Results
In total, 500 blood samples from Curaçao and 100 samples from Aruba were analysed. The mean age of donors from Curaçao was 55.1 years (range 19-75 years). Positive anti-HEV IgG donors were on average 55.3 years of age (30-73 years).
Overall, 25 (4.2%; 95% CI 2.8-6.0%) donor sera were reactive for anti-HEV IgG and one (0.17%; 95% CI 0.008-0.82%) tested positive for anti-HEV IgM. The IgG reactive samples were all HEV-RNA negative. An overview of the HEV prevalence is shown in table 1. 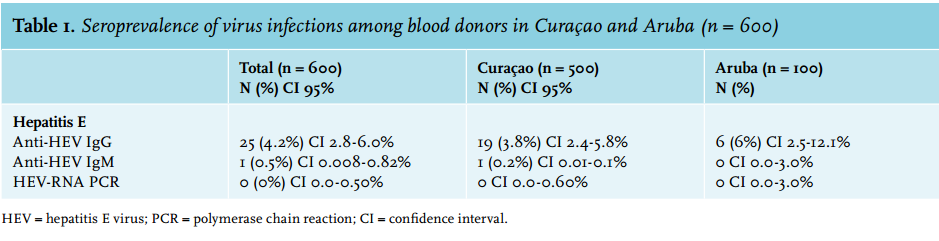
Discussion
In this combined cohort of blood donors from Curaçao and Aruba in the South Caribbean, 4.2% (95% CI 2.8-6.0%) and 0.17% (95% CI 0.008-0.82%) of the samples were found to be anti-HEV IgG and IgM seropositive, respectively. No samples were HEV-RNA positive. Based on these results, the potential risk of HEV transmission within this blood bank is very low.
The anti-HEV IgG seroprevalence and the 95% CI in our study is at the low end of the range compared with seroprevalences among blood donors in other high-income countries and surrounding regions, with ranges of 2.3-32.6%.1,8,10,11 Genotype 3 is the main cause of human HEV infections in America and Europe and can be transmitted to humans from infected animals.4,8,10 A major cause for human HEV infection is swine meat consumption; the relatively few pig farms and subsequently low swine consumption in the South Caribbean may explain the low seroprevalence.4,8
Our data may help to make a balanced decision as to whether HEV screening of blood donors should be indicated in the South Caribbean setting and whether HEV transmission among blood donors with a recent travel history to Curaçao or Aruba may be increased. Based on our findings, we do not believe that the blood banks in Curaçao and Aruba should extend their screening program for all donors to HEV. Besides that, our findings indicate that the risk of HEV transmission among Dutch donors with a recent travel history to the South Caribbean is not increased for Dutch blood recipients.
Roughly, one twentieth of blood donors have ever been infected with anti-HEV in the South Caribbean and none of the donations were viraemic. This number is not exceedingly worrying, especially since HEV infection, even in vulnerable patients, is usually self-limiting.3,4 Thus, we believe that the risk of transmission of HEV through blood donation is very small in the South Caribbean. However, it can be considered to make exceptions for selected risk groups (e.g. immune comprised patients) to test donor blood for the presence of HEV-RNA.
Our study is the first to assess the seroprevalence of HEV among blood donors in Curaçao and Aruba in the South Caribbean, although there are some limitations to be discussed. First, only anti-HEV IgM and IgG positive samples were tested for the presence of HEV-RNA. Therefore, we may have missed some positive HEV-RNA samples. However, the serological response of anti-IgM only occurs about few days after presence of HEV-RNA.3,5
Another limitation is the limited access to epidemiological data. We recommend that future sero-epidemiological studies on viral infections among blood donors should also review the patient’s past medical history, recent travel history, and lifestyle factors to determine the likelihood that test results are due to an infection with HEV. Notwithstanding these limitations, we believe that the results of our study provide a good indication of the seroprevalence and the potential risk of transmission of HEV among blood donors in the south-Caribbean.
In conclusion, the HEV seroprevalence in the present study is lower than seroprevalences among blood donors in other high income countries and surrounding regions. Globally, HEV is not included in the current standard screening programs for blood donors. Worldwide, it is debated whether screening for HEV should be performed routinely.1,3 Based on our results, there is currently is no rationale for extension of blood donation screening programs to HEV for blood donors in the South Caribbean.
REFERENCES