

KEYWORDS
Hyponatraemia, SIAD, SIADH, urea
INTRODUCTION
Hyponatraemia, which is defined as a serum sodium concentration < 135 mmol/l, is estimated to occur in up to 30-40% of hospitalised patients.1-3 Hyponatraemia can pose a therapeutic challenge, and this condition is often accepted, despite an association with poor outcomes. The 2015 multinational Hyponatraemia Registry showed that almost half of 3087 patients with hyponatraemia were discharged from hospitals with a serum sodium level of 130 mmol/l or less.1 Patients with chronic hyponatraemia (existing > 48 hours) often appear asymptomatic, but chronic hyponatraemia has been associated with cognitive deficits, an increased risk of falling, bone fractures and osteoporosis, and an increased risk of mortality.4-10
The syndrome of inappropriate secretion of antidiuretic hormone (SIADH) is a common cause of hyponatraemia, although clear prevalence data are lacking, possibly partly due to failures in diagnostic procedures.1,2 Hyponatraemia in SIADH is the result of increased retention of water due to excess antidiuretic hormone (vasopressin), and hence fluid restriction is an effective and widely accepted first-line treatment strategy.11,12 However, in patients with a high level of antidiuretic activity, strict fluid restriction may not be sufficient or cannot be applied due to another prescribed therapy or poor patient adherence. Urea is recommended as a second-line treatment for SIADH by the 2014 European Renal Best Practice (ERBP) guideline and the 2017 Dutch Practice Guideline ’Het Acute Boekje’.11,12
In the kidney, urea plays an important role in concentrating urine and acts as an osmotic diuretic. Since the diuretic property of urea is its main pharmacokinetic mechanism, it might be possible to estimate the serum sodium level after a urea dose using a patient’s urine osmolality level and total body water (TBW).13 For example, in a patient with a urine osmolality of 750 mOsm/l, 500 mmol of urea (30 g) leads to 0.66 l of electrolyte-free water excretion. If the patient has a TBW of 20 l, the new TBW is 19.34 l. The new serum sodium value can then be calculated using the formula:
In 1980, Decaux and colleagues were the first to report the use of urea as a treatment for hyponatraemia due to SIADH.14 In the years following, they published several case series and observational studies showing that urea can be successfully used for both rapid correction of acute hyponatraemia and long-term maintenance therapy for chronic hyponatraemia.15-18 Although incorporated into the European and Dutch guidelines, experience with urea therapy for SIADH is limited in the Netherlands. In this case series, our primary aim was to present data on the effectiveness and tolerability of urea as a second-line treatment in hospitalised patients with SIADH. As secondary aim, we determined whether the serum sodium levels after a dose of urea can be accurately estimated.
MATERIALS AND METHODS
This study was conducted in the Medical Centre of Leeuwarden, a 700-bed teaching hospital in the Netherlands, from December 2017 to February 2019. Formal approval was waived by the local ethical committee according to national guidelines.
In December 2017, we implemented an inpatient SIADH management protocol including urea as a second-line treatment strategy. Treating physicians from the departments of internal medicine and geriatrics were informed about the protocol and asked to report patients in need of second-line treatment to the coordinating investigator (JW). Furthermore, this investigator screened all hospitalised patients with moderate to profound hyponatraemia (serum sodium ≤ 129 mmol/l) using a notification system built into our Electronic Patient Record. In possible cases, study criteria were assessed by the coordinating investigator in collaboration with the treating physician.
Study population
Patients included in this case series were aged 18 years or older and had moderate to profound chronic hyponatraemia (serum sodium ≤ 129 mmol/l, existing for more than 48 hours) due to SIADH. A diagnosis of SIADH was made if patients met the diagnostic criteria as stated in the European guideline.11 Diagnostic procedures therefore included measurements of serum osmolality, serum glucose, urine osmolality, urine sodium, serum cortisol, serum thyroid-stimulating hormone (TSH), serum creatinine, and assessment of volume status and diuretic use by the treating physician.
Patients eligible for treatment with urea included those in whom fluid restriction was not or was minimally effective and/or in whom further restriction of fluid intake was not possible. Contra-indications for urea were moderate to severe renal impairment (chronic kidney disease stage 3b or higher) and liver failure. Patients were excluded from this case series if they received other treatment for hyponatraemia (e.g., hypertonic saline, loop diuretics combined with sodium chloride tablets), if they did not receive urea for at least two subsequent days, if they were in the intensive care unit, or if they had severe symptoms of hyponatraemia (e.g., seizures, coma) or SIADH with an expected fast-resolving cause (e.g., after discontinuation of causal medication).
The dosage of urea was determined using the guidelines’ dosage recommendation of 0.25-0.50 g/kg/day.11,12 Urea was prescribed in one dose per day or two doses when receiving 40 g/day or more. We used a urea formulation recipe known as ‘Brussels Champagne’ consisting of the following ingredients in addition to 10 g of urea: sodium bicarbonate 2 g, citric acid 1.5 g, and saccharose 200 mg. This formulation is diluted in 50-100 ml of water and can be taken orally.11,19 The previously prescribed fluid restriction was maintained in patients able to tolerate the restriction. The duration of urea therapy and continuation after discharge were determined per case, for example, by taking into account the severity and underlying cause of SIADH.
Data collection
In patient treatment
After inclusion, we documented the baseline clinical and treatment characteristics of patients. The presumed cause of SIADH was reviewed and agreed upon by the treating physician and coordinating investigator. Serum sodium concentrations were measured at admission and at the following time points in relation to the start of urea therapy: Day-2, Day-1, baseline, Day+1, Day+2, and at the end of inpatient treatment (EIT). Serum urea, creatinine, and uric acid levels were measured at baseline, Day+2 and EIT. The occurrence of side effects and overcorrection, defined as an increase in serum sodium > 8 mmol in 24 hours, were monitored. The duration of treatment and changes in urea dose were recorded. The EIT was considered to occur either at discharge or when a patient discontinued urea therapy during hospitalisation. In the case that a patient had multiple inpatient treatment episodes, only the first episode was included for this analysis.
Follow-up
If a patient was discharged with urea treatment, we asked the attending physician to perform follow-up evaluations of serum sodium levels at two and six to eight weeks after discharge. The duration of ambulatory treatment and changes in urea dose were also recorded. Restart of urea therapy during the study period was documented, both during (re)admission and ambulatory care, after reporting by the attending physician and/ or by the notification system of our Electronic Patient Record.
Estimating serum sodium
We estimated serum sodium levels for the time points Day+1 and Day+2 using the formula mentioned in the introduction section. Baseline TBW was assessed with Watson’s formula, which includes body height, weight, age, and sex.20 For the Day+2 estimation, the measured serum sodium level at Day+1 was used as the baseline serum sodium level; the baseline TBW was not adjusted.
Endpoints
The main outcomes were normonatraemia (serum sodium 135-145 mmol/l) at EIT and discontinuation of urea due to side effects. Secondary outcomes were the number of patients experiencing side effects and overcorrection. In the analysis of serum sodium estimation, the endpoint was agreement between the estimated and measured serum sodium levels, which was defined as a maximal difference of 1 mmol/l.
Data analysis
We describe continuous variables as medians and ranges or interquartile ranges (IQRs), and the Wilcoxon signed rank test was performed to compare laboratory values before and after the start of urea treatment. P-values are two-tailed, and the significance level was set at 0.05. To analyse the agreement between the method of estimating serum sodium and the gold standard of measuring serum sodium, a Bland-Altman plot was constructed, showing differences between the estimated and measured serum sodium levels plotted against the measured serum sodium level. The mean difference and 95% limits of agreement (mean difference ± 1.96 SD of the differences) were calculated using the one-sample T-test. Based on the researchers’ opinion, the maximum allowed upper and lower limits of agreement were defined beforehand as ≤ 3 mmol/l and ≥ -3 mmol/l, respectively. We used SPSS Statistics version 25 (IBM, New York, United States) and Microsoft Office 2010 (Microsoft, Redmond, United States) to analyse and display our data.
RESULTS
Study population
During the study period, 18 patients were treated with urea, of whom, 13 met the inclusion criteria (figure 1). 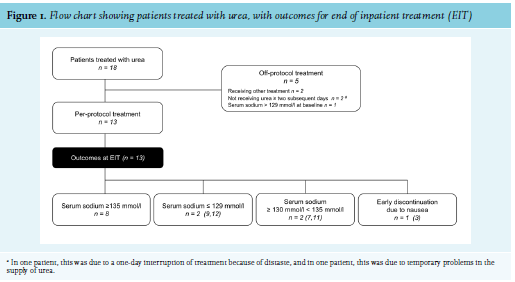
The median serum sodium level at baseline was 124 mmol/l (IQR 122-128) and the median urine osmolality at baseline was 553 mOsm/kg (IQR 478.5-743). Moderate to severe hyponatraemia was already present at admission in 11 of 13 patients, and the serum sodium levels at admission ranged from 110 to 136 mmol/l (median 125 mmol/l, IQR 117.5-126.5). Patients were treated with urea for a median of 5 days (range 2-10) in doses ranging from 10-40 g. The prescribed fluid restriction varied from 750 to 2000 cc/day. In four patients, with a fluid restriction of 1500 - 2000 cc/day, the inability to further restrict fluid intake was the main reason that urea treatment was started. Two of these patients were undergoing tube feeding for poor nutritional status.
Outcomes
Inpatient treatment
The median serum sodium level at baseline did not significantly differ from the median serum sodium level on Day-2 (124 mmol/l (IQR 122-128) versus 125 mmol/l (IQR 123-128), p = 0.53), and the level increased to 128 mmol/l (IQR 123-130) (p = 0.003) after the first day and to 130 mmol/l (IQR 127-132) (p = 0.002) after the second day of urea therapy (table 2, figure 2). During the first two days of urea treatment, the average increase in serum sodium was 2.9 mmol/day. The median serum urea level was 6.1 mmol/l (IQR 4.0-7.6) at baseline and 10.4 mmol/l (7.8-11.8) (p = 0.004) at EIT.
Serum creatinine levels at both baseline and EIT were available for 10 patients and did not significantly differ (table 2). At EIT, normonatraemia was observed in 8 of 13 patients (62%). Two patients (15%) had a serum sodium level between 130 and 135 mmol/l, and two patients (15%) had a serum sodium level of ≤ 129 mmol/l. In patient 3, urea was discontinued due to nausea after four days of therapy, and at that time, the patient had a serum sodium level of 129 mmol/l (figure 1). Six patients reported light to moderate intake difficulties due to the taste of urea, and one of these patients also reported light nausea that was likely related to urea. All of these patients were able to continue urea therapy. Overcorrection was not observed.
Follow-up
In patients 5 and 8, urea was restarted during admission because of a decline in serum sodium. In patient 11, urea was restarted during readmission for hyponatraemia. A total of eight patients received ambulatory urea treatment with a duration ranging from 13 days to > 5 months. This included patients 5, 8, and 11. In the five other patients, urea was directly continued after the first inpatient treatment episode, including both patients with serum sodium levels ≤ 129 mmol/l at EIT. Both patients received a higher dose during ambulatory treatment and showed normalisation of serum sodium during follow-up. The median serum sodium level at two weeks of follow-up was available for six patients and was 134 mmol/l (range 132-141). The median serum sodium level at six to eight weeks of follow-up was available for five patients and was 134 mmol/l (range 128-140).
Estimating serum sodium
Serum sodium levels were estimated for 25 measurements, with one missing value for Day+2. The Bland-Altman plot shows the differences between the estimated and measured serum sodium levels (figure 3). Thirteen of 25 (52%) estimated serum sodium levels were in agreement with the measured serum sodium level. The upper limit of agreement was 4.49 mmol/l, and the lower limit of agreement was -4.17 mmol/l, hence exceeding the defined maximum allowed limits of ≤ 3 mmol/l and ≥ -3 mmol/l.
DISCUSSION
We introduced urea as second-line treatment for hyponatraemia due to SIADH and found it to be an effective inpatient treatment strategy; most patients showed a direct increase in serum sodium after urea commencement, and 8 of 13 patients achieved normonatraemia at the end of inpatient treatment. Tolerability can be reduced due to an unpleasant taste of urea, but in most patients, this did not lead to discontinuation. Serious side effects were not observed.
In many hospitalised patients with hyponatraemia due to SIADH, effective management is not achieved.1 Fluid restriction, in addition to treating the underlying condition, is the first-choice treatment; however, fluid restriction may not be sufficient, cannot always be applied due to the use of other therapy, or fails due to poor adherence. Pharmacotherapeutic interventions for SIADH may have disadvantages such as cost and lack of outpatient reimbursement (tolvaptan), concerns regarding side effects (tolvaptan, demeclocycline), or very limited evidence on their efficacy (oral sodium chloride combined with loop diuretics).11,21,22 Urea recently attracted global interest due to evidence of its efficacy and safety from several observational studies.23-25 Despite the inclusion of urea in treatment guidelines,11,12 urea remains an intervention that is not formally registered based on an adequate balance of benefits and risks. Structured reports on the efficacy and safety of urea in SIADH are therefore necessary to obtain a better estimation of its added therapeutic value.
All of our patients had moderate to profound hyponatraemia (median serum sodium at baseline 124 mmol/l) and were not effectively treated with fluid restriction alone, as reflected in the median serum sodium level of 125 mmol/l at two days prior to baseline. We observed an average increase in serum sodium of 2.9 mmol/day during the first two days of urea treatment, which is consistent with findings in American and Australian observational studies of hospitalised patients with hyponatraemia.23,24 It cannot be fully ruled out that recovery of the underlying cause of SIADH also improved serum sodium levels, for example, in patients with pulmonary infection. However, we believe that this was not a major factor in serum sodium increase, since all of these patients showed no increase in serum sodium in the 48 hours before initiation of urea therapy. We found a higher proportion of patients with normalisation of serum sodium than among the urea-only treated American patients (8 of 13 (62%) versus 4 of 12 (33%), respectively).23 Distaste was also frequently reported in our population in 7 of 13 patients (54%), whereas Lockett et al. reported distaste in 7 of 69 patients (10.1%).24 The higher proportion of both efficacy and tolerability outcomes in our case series, as compared to the recent observational studies, can possibly be explained by our prospective design, in which serum sodium and side effects were actively monitored.
Despite the use of the more palatable ‘Brussels Champagne’ formulation,11,19 distaste was still frequently reported. Urea was discontinued early in one patient due to nausea. She weighed 58 kg and received a dosage of 30 g/ day, which is on the upper limit of the dosing directive, possibly contributing to intolerance. It is possible that tolerability can further be improved by splitting the dose, by diluting urea in orange juice and by taking urea after a meal.
Urine osmolality (mOsm/kg) is a parameter of antidiuretic activity and is useful in the management of SIADH.26,27 For example, Winzeler et al. found that urine osmolality and urine sodium were significantly associated with nonresponse to fluid restriction. Optimal cut-off values predictive for nonresponse were ≥ 500 mOsm/kg for urine osmolality and ≥ 130 mmol/l for urine sodium.26 Most of our patients had a urine osmolality level ≥ 500 mOsm/kg. In two of our patients with a very high urine osmolality level of ≥ 700 mOsm/kg, urea dosage had to be increased to 0.5g/kg/day to increase serum sodium. During urea therapy, fluid restriction was continued in patients able to be restricted. This was done because our patients were considered to have more severe SIADH as reflected by a high urine osmolality level and prior nonresponse to fluid restriction alone. Most of our patients had a moderate fluid restriction of 1000-1250 cc/day. In our hospital, a severe fluid restriction is not often prescribed, due to poor patient acceptance and also healthcare providers’ unfamiliarity with the management of more severe SIADH. Nervo et al. successfully treated ambulatory patients with urea as single therapy without fluid restriction. Most of their patients received a higher urea starting dose of 30 g and their patients had a lower mean urine osmolality level (452 mOsm/kg vs 604 mOsm/kg in our population).25
In the management of hyponatraemia, particular attention is also necessary to prevent an excessively rapid correction and thereby osmotic demyelination syndrome, a rare but feared complication. Soupart et al. showed in animal models that urea therapy may additionally protect from osmotic demyelination.28 Overcorrection during urea treatment has been observed in patients in intensive care units receiving a high dosage of urea (0.5-1.0 g/kg/day),16,17 but not in our study or in other recent studies.23-25 To further guide dosing, the ability to estimate the effect of urea on serum sodium might be helpful. As described by Sterns et al., urea mainly acts as an osmotic diuretic and serum sodium levels were estimated based on this property.13 Thirteen of 25 (52%) estimated serum sodium values were in agreement with the measured serum sodium level. However, the 95% limits of agreement did not meet our defined criteria of ≤ 3 mmol/l and ≥ -3 mmol/l, hence, we cannot conclude that this method is accurate. A major limitation of this analysis was the small sample size; therefore, additional data are needed. We had a possible outlier (6 mmol/l difference) that we could not readily explain, but even after excluding this outlier from the analysis, the limits of agreement were not met. The method might be oversimplified; it has been shown that urea not only promotes water excretion, but also decreases natriuresis.13,29 Furthermore, the method assumes stable fluid and food intake, which were not fully controlled for in this study and are also not fully controlled in real-life practice, which may limit the use of this method. The main limitations of this study are its small sample size and uncontrolled design. The strengths of our study are the prospective nature of data collection, patient follow-up in a real-life practice setting, and applying strict diagnostic criteria for SIADH.
In conclusion, this study adds to the evidence that urea is an effective second-line treatment strategy for hospitalised patients with hyponatraemia due to SIADH. Distaste and nausea were reported side effects in our population, but, for the most part did not lead to early discontinuation. After the inclusion of urea treatment in the 2014 European and the 2017 Dutch practice guidelines, these are the first data from the Dutch population, and to our knowledge, this is only the second study on this subject to perform prospective evaluation of data. Additional data are needed to assess the long-term effectiveness and tolerability of urea treatment.
ACKNOWLEDGEMENTS
31th Annual Meeting of The Netherlands Association of Internal Medicine, 24 April 2019, Maastricht, the Netherlands. Abstract O4.06 ‘Urea as second-line treatment for hyponatremia due to the syndrome of inappropriate antidiuretic hormone secretion: a case series involving 13 in-hospital patients’.
Scientific Spring Meeting of Dutch Society for Clinical Pharmacology and Biopharmacy, 12 April 2019, Rotterdam, the Netherlands. No abstract available online.
REFERENCES