

KEYWORDS
Anabolic androgenic steroids, performance and image enhancing drugs, hepatic adenoma, illicit drug use
INTRODUCTION
The use of anabolic androgenic steroids (AAS) by amateur strength athletes is widespread, with an estimated prevalence rate of 6%.1 AAS are often used in cycles and are comprised of an injectable (intramuscular) testosterone ester and one or two other AAS types. Between cycles, no AAS are used, allowing the pituitary-gonadal axis to recover. However, about 5% use AAS continuously. In addition to AAS, other performance and image enhancing drugs (PIEDs) are commonly used, such as clenbuterol or growth hormone.2
Side effects are reported by virtually all users of AAS and include acne, gynecomastia, agitation, decreased libido, erectile dysfunction, and depressed mood.2 Illegally obtained medications such as isotretinoin, tamoxifen, and human chorionic gonadotrophin are used to combat side effects. Little is known about the long term negative health effects of AAS use, but these likely include cardiovascular toxicity3 and hypogonadism.4 We present a case of a strength athlete who used extreme dosages of AAS and suffered a spontaneous haemorrhage of a hepatic adenoma.
CASE STUDY
A 27-year-old muscular male with an otherwise unremarkable medical history presented at the emergency room with sudden severe abdominal pain in the right upper quadrant. Computed tomography of the abdomen revealed a 15 cm large subcapsular liver haematoma in a lesion typical of adenoma. Two smaller adenomas were present in the liver. There was evidence of active bleeding (see figure 1). Fluid resuscitation and blood transfusion were performed in the intensive care unit. A branch of the right hepatic artery was coiled to control the persistent bleeding and prevent multi-organ failure, as well as the need for emergency laparotomy. Continuous venovenous haemofiltration was necessary for several days because haemorrhagic shock had led to acute tubular necrosis.
The patient admitted he had been using AAS for the past five years. Before this, he had struggled with cocaine, cannabis, and alcohol addiction as well as depression. He started using a limited amount of AAS in cycles separated by several months. This was followed by shortened intervals, an increase in AAS types and dosages, and for the last three years he had been alternating cycles with a high maintenance dose (‘blast and cruise’). His cycle prior to the haemorrhage comprised more than 10 different AAS types. During the peak of this cycle he injected 60 ml of AAS per week, which is about 15,000 milligrams of testosterone equivalents. In addition to AAS, he was concurrently using growth hormone (3.5 IU daily), clenbuterol, levothyroxine, tamoxifen, and anastrozole, as well as protein, vitamin, and mineral supplementations. The patient explained his escalated AAS use was due to a high level of ambition and a deep-seated distortion of his self-image. 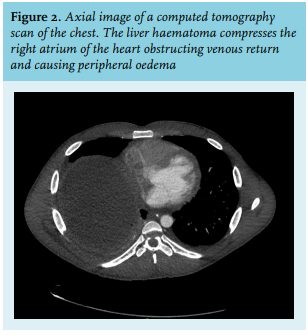
Almost two months after admission, the patient was discharged. During hospital admission, the patient did not use AAS and his testosterone concentration gradually declined from 195 nmol/l to 12 nmol/l. Gonadotropin levels were undetectable at all times. Replacement therapy with an injectable blend of testosterone esters was started on an outpatient basis when the testosterone concentrations became insufficient, with a limited issue of vials per prescription. The patient was followed-up alternately by an endocrinologist and addiction specialist at short intervals to survey hormone therapy, address the AAS addiction and muscle dysmorphia, and prevent relapse of AAS use. With magnetic resonance imaging, the liver adenoma was not visible anymore. One year after the hospital admission, the patient is in good health and has not used AAS or other illicit drugs again.
DISCUSSION
The occurrence of hepatic adenomas is very rare among men not using AAS, with an estimated incidence of less than 1 per million.5 Spontaneous haemorrhage is a known complication of hepatic adenoma. The association between androgens and hepatic adenomas was noticed previously in patients treated for hereditary angio-oedema6 and Fanconi anaemia7 and has been reported in users of AAS as well.8 The exact incidence of hepatic adenomas among users of AAS is unknown, but the majority is probably unnoticed. AAS presumably induce hepatic adenomas through the action of oestrogens derived from aromatization of AAS.9 There appears to be a dose-dependent relationship between sex hormones and liver tumour occurrence, which may explain the size and spontaneous haemorrhage of the adenoma in our patient.
The presented case illustrates the possible dramatic course of AAS addiction. Although the patient started with separate AAS cycles, he eventually used AAS continuously. The applied dosages in the months before the liver haemorrhage were astronomical with 15,000 mg of testosterone equivalents per week, which by far is the most reported by an AAS user based on our experience in the AAS clinic – an outpatient clinic for past users of AAS in the Netherlands. In an earlier survey, the average dose of AAS equivalents used during a cycle was approximately 1000 mg per week, which is already a tenfold physiological amount.10
As many as 30% of AAS users at some point develop a certain degree of AAS addiction. Addiction to other recreational drugs and muscle dysmorphia were risk factors in our patient that predisposed him to AAS addiction.11 Treating a patient for AAS addiction should be a joint effort between an endocrinologist and addiction specialist. In this patient, due to prolonged suppression of the pituitary-gonadal axis, recovery of normal endogenous testosterone was unlikely in the short term and there was a high likelihood of a permanent post-AAS-hypogonadism.2 Symptoms of testosterone deficiency would increase the urge to use AAS again. Therefore, we started testosterone replacement therapy as soon as hypogonadism occurred, which took several months due to the long half-life of certain testosterone esters, such as decanoate. Health parameters such as liver enzymes, haematocrit, and cholesterol were monitored. An addiction specialist with an interest in AAS addiction applied cognitive behavioural therapy. Although this treatment was successful in our patient in the first year, the risk of relapse remains high.
DISCLOSURES
All authors declare no conflicts of interest. No funding or financial support was received.
REFERENCES