

KEYWORDS
Mood disorders, lithium, renal concentrating ability, nephrogenic diabetes insipidus, glomerular filtration rate, chronic kidney disease.
INTRODUCTION
Bipolar disorder (BD) is a common mental illness that is characterized by recurrent episodes of major depression, mania, and hypomania.1 The estimated lifetime prevalence of BD among adults worldwide is 1-3% and the mean age of onset is approximately 20 years.2,3 Lithium has become the most effective and widely prescribed drug for mood stabilization in patients with mood disorders.4 Until now, lithium remains a mainstay of treatment for BD, especially for acute mania and maintenance therapy. Furthermore, lithium has been shown to reduce the risk of suicide in patients with BD.5 However, lithium exposure has been associated with several forms of renal injury. The most common renal side effect is nephrogenic diabetes insipidus (NDI). Although initially reversible, NDI can become irreversible with continued lithium use.6 As a result, patients are not able to limit their urine output and must maintain a high fluid intake to avoid volume depletion. Since volume depletion increases proximal reabsorption and serum lithium levels, NDI places the patient at an increased risk for acute lithium intoxication. Furthermore, long-term lithium therapy has been associated with chronic kidney disease (CKD).7 However, available data do not allow firm conclusions of the magnitude and clinical relevance of lithium-induced CKD.
Aims of the study
The aim of our cross-sectional study is to evaluate the prevalence and severity of NDI and CKD in lithium-treated patients. This cohort will be followed prospectively and the results will be published separately to provide insight intp the association of renal concentrating ability and estimated glomerular filtration rate (eGFR) in patients on lithium maintenance therapy.
MATERIAL AND METHODS
Sample
The design of the study is shown in figure 1. The study was divided into two phases. For phase I, patients with a mood disorder treated at the outpatient psychiatry clinics of the Canisius Wilhelmina Hospital (CWZ), Nijmegen or GGZ inGeest Mental Health Center, Amsterdam, the Netherlands were screened between November 6th, 2012 and August 27th, 2013. Since we aimed to include 100 patients in phase II and estimated that 50% of patients would be willing to participate, the target population for phase I of the study was 200 patients.
Patient selection was performed based on the following inclusion criteria: 1) age ≥ 18 years, 2) diagnosis of BD or unipolar depression, and 3) current lithium treatment. Exclusion criteria included: patients 1) with a physical or psychiatric unstable condition, 2) who were hospitalized at the time of screening, or 3) who were unable to provide written informed consent. Two hundred forty-six patients were screened, provided with written information and invited by their treating psychiatrist to participate in phase I of the study. Informed consent was obtained from 201 patients (81%). Participants were asked to visit the outpatient clinic once for 30-45 minutes. Prior to this visit, they were asked to fill out a questionnaire including demographic characteristics (sex, age, race); psychiatric diagnosis; lifetime course of mood disorder; duration, dose and interruptions of lithium use; lithium intoxications; and alcohol and smoking habits. During the visit at the outpatient clinic, questionnaire information was discussed and the following additional data was collected: medical history, complaints of polyuria, thirst and polydipsia, micturition complaints, and current use of medication. This information was verified with the respective patient’s medical and pharmacy records. Body height, weight, and waist circumference were recorded and blood pressure and heart rate were measured with an automatic blood pressure monitor (Omron 705IT). In addition, blood and urine samples were collected for measurements of serum urea, creatinine, sodium, potassium, calcium, phosphate, bicarbonate, osmolality, albumin, glucose, glycosylated haemoglobin (HbA1c), total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, thyroid stimulating hormone and free thyroxin, 25-OH vitamin D, parathyroid hormone and urine dipstick (pH, glucose, erythrocytes, leukocytes, nitrite), creatinine, and albumin. Finally, additional blood and urine samples were collected and stored for future research.
Participants of this first phase were also invited to take part in phase II of the study, which included an additional 1-desamino-8-D-arginine vasopressin (dDAVP) test to determine maximal urine osmolality as a measure of renal concentrating ability. To this end, 134 patients participating in phase I were screened for eligibility to participate in phase II of the study between November 30th, 2012 and July 8th, 2013. The inclusion criteria were identical. The following additional exclusion criteria were applied: inability to comply with water restriction, moderate to severe CKD (MDRD-eGFR ≤ 45 ml/min/1.73m2 ), hyponatraemia (sodium < 130 mmol/l), hypo- and hyperkalaemia (potassium < 3.0 or > 5.5 mmol/l), severe hypercalcemia (calcium > 2.80 mmol/l), hyperglycaemia (glucose > 10.0 mmol/l), established primary polydipsia or central diabetes insipidus, history of Sjögren syndrome, amyloidosis, sickle cell anaemia or previous treatment with ifosfamide, current treatment with desmopressin or demeclocycline, or pregnancy. Informed consent was obtained from 100 patients. The dDAVP test was performed during an additional 6-hour visit to the outpatient clinic. We followed the protocol of Tryding et al. with slight modifications.8 Fluid intake was restricted from 22.00 hrs. the evening prior to the test. On the following morning, a fluid intake of 250 ml and a light breakfast were allowed. During the test, an additional fluid intake of 250 ml was allowed. At baseline, we recorded the presence of polyuria, thirst, polydipsia, micturition frequency and measured body height, weight, blood pressure, and heart rate. Blood samples were collected for measurements of serum creatinine, sodium, potassium, calcium, osmolality, glucose, and lithium levels. Additionally, urine samples were collected of measurements of creatinine, sodium, and osmolality. After voiding, 40 µg dDAVP was administered intranasally. Water intake, body weight, blood pressure, and heart rate were determined at six hours after administration of dDAVP and additional urine samples were collected at four and six hours after administration of dDAVP. Maximal renal concentrating ability was determined by measuring osmolality in the collected urine samples. The dDAVP test was not performed in two patients. One patient was scheduled for coronary artery bypass graft and another patient was excluded because of hyperglycemia and glycosuria.
Definitions
For the purpose of these analyses, the following definitions were used: duration of lithium use (total treatment duration with subtraction of periods of discontinuation of lithium); hypertension (systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg and/or treatment with antihypertensive drugs); dyslipidaemia (HDL cholesterol < 1.04 mmol/l, LDL cholesterol > 4.14 mmol/l, total cholesterol > 6.22 mmol/l, and/or treatment with lipid-lowering drugs);9diabetes mellitus (random plasma glucose ≥ 11.1 mmol/l, HbA1c ≥ 48 mmol/l, and/or treatment with oral glucose-lowering drugs); cardiovascular comorbidity (history of cardiovascular events and/ or treatment with acetylsalicylic acid, dipyridamole, or clopidogrel). Patients with a maximal urine osmolality 600-800 mOsmol/kg and < 600 mOsmol/kg were diagnosed as lithium-induced impaired renal concentrating ability and NDI, respectively. eGFR was calculated from serum creatinine values using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.10 To estimate the proportion of cases with different degrees of CKD, patients were classified according to the Kidney Diease: Improving Global Outcomes 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease.11
Statistical analysis
Continuous variables are reported as median and inter-quartile range (IQR) or mean and standard deviation (SD). We compiled two separate directed acyclic graphs (DAGs), by using DAGitty software (http://www.dagitty. net), to select a minimal sufficient adjustment set of variables that would allow the identification of an unconfounded effect of duration of lithium treatment on our two outcome measures, e.g. maximal urine osmolality and eGFR. The DAGs were built by identifying all known factors affecting duration of lithium treatment, maximal urine osmolality, or eGFR. Variables in the minimal sufficient adjustment set blocked all non-causal but not the causal pathway between duration of lithium treatment and maximal urine osmolality or eGFR, and included current age, gender (male versus female), race (Caucasian versus other), centre (Amsterdam versus Nijmegen), psychiatric diagnosis (bipolar disorder versus other), and psycho-pharmacological comedication (anticonvulsants, antidepressants, antipsychotics, and sedatives). With linear regression analysis, we studied the independent effect of duration of lithium therapy on maximal urine osmolality or eGFR as dependent variables adjusted for all variables that were identified for the minimal sufficient adjustment set (age, sex, race, center, psychiatric diagnosis, and psycho-pharmacological comedication). Since cigarette smoking, body mass index, hypertension, dyslipidaemia, diabetes mellitus, maximal urine osmolality, or eGFR and cardiovascular comorbidity are likely intermediate variables in the pathway between duration of lithium treatment and maximal urine osmolality or eGFR, our multivariable models did not include these variables as potential confounders. Any association was considered statistically significant if the p-value was less than 0.05.
The study protocols were approved by the central ethics committee on research involving human subjects and the local ethics committees of the participating sites. All patients gave written informed consent before inclusion in the study. The study was performed in accordance with the Declaration of Helsinki.
RESULTS
See tables 1 - 6
Patient characteristics
The patients screened, invited, participated, and evaluated in phase I and II of the study are shown in figure 1. Baseline demographic and clinical characteristics are shown in table 1. Ninety-eight patients participated in phase II of the study, 37 males and 61 females. The mean age was 51 years (SD: 12). The majority of these patients (86%) had a diagnosis of BD. Patients had been treated with lithium for median 7 years (IQR: 4-15). Twenty-two patients (22%) were on lithium for > 15 years. According to the questionnaire, medical records and examination at the psychiatry outpatient clinic, 24 patients (25%) were obese (BMI > 30 kg/m2 ); 33 patients (34%) smoked cigarettes; 49 patients (50%) had hypertension, with 30 of them (31%) being treated with antihypertensive drugs; 37 patients (38%) had dyslipidaemia, with 8 of them (8%) being treated with statins; 3 patients (3%) had diabetes mellitus type 2, all of them being treated with oral blood-glucose lowering drugs; and 7 patients (7%) had experienced a cardiovascular event.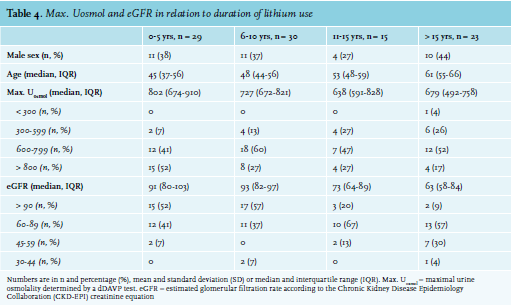
Adverse effects, urine osmolality and renal function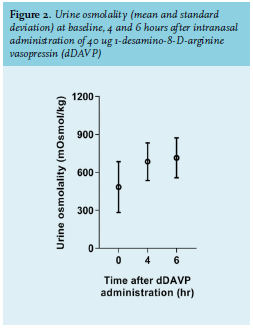
The median eGFR in our study population was 84 ml/min/1.73 m2 (IQR: 68-95). Twelve patients (12%) had an eGFR < 60 ml/min/1.73 m2 and 10 patients (10%) had albuminuria. Of the total population, 19 patients (19%) had CKD. Patients with an eGFR < 60 ml/ min/1.73 m2 were older than patients with an eGFR ≥ 60 ml/min/1.73 m2 (median age 65 vs. 50 years, p < 0.001), had been treated with lithium longer (median 21 vs. 7 years, p = 0.003), had hypertension more often (100% vs. 44%, p = 0.03), and had a lower maximal urine osmolality (median 542 vs. 745 mOsmol/kg, p < 0.001). As expected, both groups of patients had similar serum lithium concentrations (median 0.76 vs. 0.71 mmol/l, p = 0.64), but were treated with a lower lithium dose (median 600 vs. 800 mg/day, p = 0.007).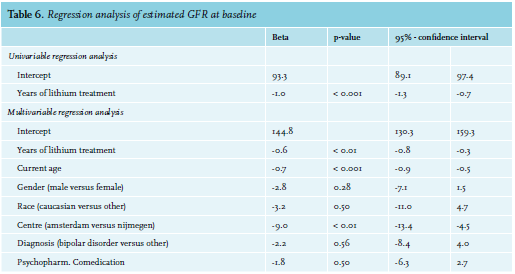
As shown in table 5, the univariable regression model showed a negative association between the duration of lithium therapy and maximal urine osmolality (B = -7.4, 95% confidence interval (CI) -10.3 to -4.6, p < 0.001). After correction for the confounding effects of age, sex, race, center, psychiatric diagnosis, and psycho-pharmacological comedication, duration of lithium therapy remained independently and inversely associated with maximal urine osmolality (B = -6.1, 95%-CI -9.4 to -2.9, p < 0.01). This means that after adjusting for the set of potential confounders, each additional year of lithium therapy results in a loss of maximal renal concentrating ability of 6.5 mOsmol/kg.
A similar model was built for the association between duration of lithium therapy and renal function (table 6). The univariable regression model showed a negative association between the duration of lithium therapy and eGFR (B = -1.0, 95%-CI -1.3 to -0.7, p < 0.001). After correction for the confounding effects of age, sex, race, center, psychiatric diagnosis, and psycho-pharmacological comedication, each year of lithium therapy leads to an additional decline of eGFR of 0.6 ml/min/1.73 m2 (95%-CI -0.8 to -0.3, p < 0.01). Of note, in the multivariable analysis, centre was also associated with a lower eGFR (9.0 ml/min/1.73 m2 , 95%-CI -13.4 to -4.5, p < 0.01).
DISCUSSION
This study shows that a majority of lithium-treated patients had complaints of polyuria, thirst, and polydipsia. The data also demonstrate that almost one-fifth of patients did have a lithium-induced NDI. Surprisingly, polyuria, thirst, and polydipsia did not predict NDI as determined by a dDAVP test. Our study does add that a questionnaire of symptoms alone cannot be used to accurately identify patients with an impaired renal concentrating ability.12-14 In addition, maximal urine osmolality could not be predicted by the urinary creatinine concentration measured in an urine sample collected after an overnight fluid restriction. Furthermore, duration of lithium therapy was independently and significantly associated with a decline in maximal urine osmolality. A disturbance in renal concentrating ability was already observed within five years of lithium treatment and the prevalence of an impaired renal concentrating ability increased with the duration of lithium maintenance therapy. These findings are in agreement with other studies which included patients with a comparable age, duration of lithium therapy, and renal function.15-17
In addition, we observed a prevalence of CKD exceeding 10%. Multivariable linear regression analysis showed that eGFR was associated with duration of lithium treatment. Several earlier studies reported no significant change18-23 or only a slight deterioration in renal function with long-term lithium exposure.13,17,24 In contrast, recent studies have shown a marked increase in the prevalence of CKD (eGFR < 60 ml/min/ 1.73 m2), especially in patients treated for many years.7,25-28 Moreover, several authors have shown that long-term lithium therapy causes end-stage renal disease in a small proportion of patients.29-34 One possible explanation for these apparently conflicting results is that earlier studies often relied on serum creatinine values alone which might have resulted in an underestimation of the prevalence of CKD. In addition, earlier studies predominantly reported on patients who were treated with lithium < 10 years. However, it may take decades before lithium-treated patients develop CKD. Our data show that duration of lithium treatment is independently, significantly and inversely associated with eGFR. This finding is more relevant, since CKD is associated with an increased risk of cardiovascular morbidity and mortality.35 We therefore agree with Kripalani et al. that monitoring of cardiovascular risk factors is important to prevent cardiovascular events in this patient population.36
We studied the independent effect of duration of lithium therapy on maximal urine osmolality and eGFR as dependent variables adjusted for age, sex, race, center, psychiatric diagnosis, and psycho-pharmacological. However, our multivariable models did not include cigarette smoking, body mass index, hypertension, dyslipidemia, diabetes mellitus, maximal urine osmolality, or eGFR and cardiovascular comorbidity. We therefore performed an additional analysis including these potentially causal risk factors which confirmed that duration of lithium therapy was independently, significantly and inversely associated with maximal urine osmolality and eGFR.
It should be noted that a significant decline in renal concentrating ability was observed in 14 out of 29 individuals (48%) treated with lithium for ≤ 5 years. This observation is in accordance with findings of others who showed that lithium acutely and, with prolonged lithium exposure, progressively impairs renal concentrating ability. In healthy subjects, even a single oral dose of lithium carbonate has been shown to impair the antidiuretic response to hypertonic saline infusion, resulting in an increased urine output (median 3.7 vs. 1.9 ml/min, p = 0.05).37 In addition, a 4-week treatment of healthy volunteers with lithium carbonate also significantly reduced renal concentrating ability (mean maximal urine osmolality 960 vs. 1040 mOsmol/kg, p < 0.01).38 In patients with mood disorders, longer exposure to lithium results in a progressive decline in renal concentrating ability.39 Although this inability to adequately concentrate urine is initially reversible,40-43 prolonged lithium exposure has been demonstrated to result in irreversible NDI.44 It is not clear at which point lithium-induced NDI becomes irreversible. In most patients, a decrease in eGFR below 60ml/min/1.73 m2 became apparent after treatment with lithium for at least 10 years. This observation suggests that a decline in renal concentrating ability likely precedes a decrease in eGFR. Our prospective study will enable us to investigate the predictive value of impaired renal concentrating ability with regard to the risk of developing lithium-induced CKD.
On the other hand, it can be argued that approximately half of patients had a normal renal concentrating ability after ≤ 5 years of lithium therapy. This suggests that there might be differences between individuals in sensitivity of the renal collecting ducts to lithium toxicity. We propose that future studies should focus on these differences.
Our study has several strengths. Besides lithium use and eGFR, we also report on albuminuria, prevalences of additional risk factors, and comorbidities like obesity, smoking, hypertension, diabetes mellitus, and cardiovascular morbidity and use of comedication. In addition, we report maximal renal concentrating ability determined by the current gold standard, a dDAVP test in a relatively large population. Only two similar studies have been published since 1990 reporting on renal concentrating ability in lithium-treated patients determined by a dDAVP test.17,45 Our study also has some limitations. We performed a cross-sectional analysis and are therefore unable to ascertain causality between our covariates and renal concentrating ability or eGFR. Second, only patients currently treated with lithium were included in our study. Since lithium therapy may have been discontinued in patients who had developed a clinically significant reduction in renal concentrating ability or eGFR, bias towards including individuals with a more favourable course cannot be excluded. In this respect, the differences between the centres Amsterdam and Nijmegen are notable. This suggests unreported differences between the patient populations from both centers. Alternatively, we cannot exclude a difference between centres in back-referral to primary care. Furthermore, our study has a relatively small sample size resulting in an impaired statistical precision and lacks a control group.
CONCLUSION
In conclusion, our results demonstrate that both renal concentrating ability and eGFR are significantly and inversely associated with duration of lithium treatment. In addition, a significant decline in renal concentrating ability was observed in half of the individuals treated with lithium for ≤ 5 years, whereas in most patients, a decrease in eGFR below 60 ml/min/1.73 m2 became apparent after treatment with lithium for at least 10 years. This observation suggests that a disturbance in renal concentrating ability precedes the development of CKD. Further research is needed to elucidate if abnormalities in renal concentrating ability predict the development of lithium-induced CKD. If so, renal concentrating ability can be used to select patients at high risk for CKD, which is not only worthwhile for selection of patients for increased surveillance, but also for future research, including placebo-controlled trials to investigate the benefit of amiloride to prevent lithium-induced CKD.
DISCLOSURES
This project received support from a grant from the Dutch Kidney Foundation (CPI-12.01). The funding source had no role in the collection, analysis, or interpretation of the data or in the decision to submit the manuscript for publication. We thank all patients for their participation and all physicians and nurses of the participating centres for their help. The authors declare no conflicts of interests.
REFERENCES