

KEYWORDS
Clinical pathway, emergency department, epidemic, influenza, point-of-care-testing
BACKGROUND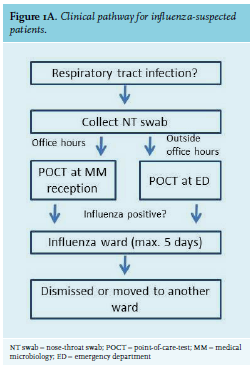
MATERIALS AND METHODS
Ethical approval
Due to the retrospective and anonymous nature of this study, ethical review was waived by the Ethical Committee Brabant (no. NW2018-15).
Setting
The JBH is a teaching hospital with 575 beds. The ED is equipped with 12 beds that are suitable for influenzasuspected patients. In the Netherlands, free influenza vaccination is available for people over 60 years of age, people with chronic diseases, and health care workers. Vaccine coverage was and 15% and 19% for healthcare workers in the JBH during the epidemics of 2016-2017 and 2017-2018, respectively.8
PCR tests
The Cobas Liat system is a PCR-based test for a single sample with a turnaround time of 20 minutes and approximately two minutes hands-on time (Cobas Liat, Roche Molecular Diagnostics, Mannheim, Germany). We used the combined influenza A/B/RSV assay; RSV is not included in the present study. Prior to commissioning the test, a laboratory validation process was performed using 14 clinical samples and 10 samples from a proficiency testing panel. The test results were compared to a Panther Fusion influenza A/B/RSV test (Hologic, Zaventem, Belgium) as well as in-house PCRs. Both commercial systems were more sensitive compared to the in-house PCRs (in-house 72%, Panther 83%, Liat 94% of positive test samples detected; see Supplementary Materials)*. During the 2016-2017 influenza epidemic, the standard of care was a laboratory-developed real-time PCR to detect influenza A/B on the BD MAX System (Becton Dickinson, Erembodegem, Belgium).
Clinical implementation and training
Six key users (three microbiology technicians and three ED nurses) were trained by Roche, after which ED nursing staff (n = 50) and receptionists (n = 5) were trained by the technician key users. Training included a Microsoft PowerPoint presentation, a pipetting exercise, running a demo sample, and a clinical sample. Further details are provided in the Supplementary Materials.
Samples
Two nose-throat (NT) swabs in Eswab medium (Copan Diagnostics, Brescia, Italy) were collected from each ED patient fulfilling the case definition: temperature ≥ 38.0 °C and symptoms of acute respiratory tract infection. One swab was used for POCT at the microbiology reception desk or ED, depending on time and day: during office hours, influenza testing was performed by laboratory receptionists; during weekends and nightshifts, ED nurses performed the test. The second NT swab was used for a confirmatory Panther Fusion test at the laboratory. Samples were stored at room temperature until collection during the next day of business. We considered the sensitivity of the PCR test to detect respiratory viruses using NT swabs as compared to nasopharyngeal swabs acceptable based on a number of studies including hundreds of patients.9-11 As available literature did not report inferior test performance of the Cobas Liat POCT in daily hospital practice,5 we decided to perform pairwise testing on a limited number of samples, followed by random pairwise testing (total n = 121). In case of a discrepant test result (one NT swab positive, while the other NT swab from the same patient was negative), the two swabs were reciprocally tested.
Temporary influenza ward
A separate ward was set up for all influenza-positive patients, except for those requiring special care at another ward (e.g., intensive care, cardiac care, haematology, oncology, or children’s ward; other types of patients were eligible for admission to the influenza ward). A capacity of 15 beds was calculated based on numbers of the 2016-2017 influenza season. Patients were admitted into single rooms if available; if not, they were admitted in cohort nursing according to influenza type A or B. In accordance with the number of days that patients have to be nursed in isolation, these patients were either dismissed or moved to another ward after five days, a rule that was strictly adhered to. A weekly multidisciplinary gathering (attending geriatrics doctor, ward nurse, planning nurse, manager, pulmonologist, internal medicine specialist, clinical microbiologist, infection control nurse) was set up to evaluate the process and adjust if necessary. The pathway including POCT and influenza ward was operational as soon as logistically possible after the start of the influenza season, which was on January 8th, 2018.
Statistics
Data were retrospectively collected for the influenza epidemics of 2016-2017 and 2017-2018 according to the national crossing of epidemiologic thresholds (week 48, 2016 - week 10, 2017 and week 50, 2017 - week 15, 2018). Data were gathered from the electronic patient record system Hix (ChipSoft), and the laboratory information system MOLIS (CompuGroup Medical). Only patients admitted via the ED were included in the analyses. Data including all patients is presented in the Supplementary Materials. Analyses were performed using R studio built under R version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria). A p-value of < 0.05 was considered to be statistically significant. Normal distribution of each data set was evaluated using a Shapiro-Wilk test. All data was nonparametric and analysed using a Mann-Whitney U-test; results are presented as median [interquartile range (IQR)].
Cost analysis
A rough estimate of the costs of this clinical pathway is based on the costs of laboratory and clinical aspects as well as costs based on the of length of stay, the number of hospitalisations of influenza-positive patients and the number of unnecessary isolations during the 2017-2018 season compared to the previous. Details are provided in the Supplementary Materials.
RESULTS
The Dutch National Institute for Public Health and the Environment (RIVM) reported an influenza epidemic lasting 15 weeks in 2016-2017 and 18 weeks in 2017-2018, with a 20-year average of nine weeks in the Netherlands.12 During the entire 2016-2017 season, influenza A(H3N2) dominated and influenza A(H1N1)pdm09 and B were only sporadically detected. The 2017-2018 epidemic was dominated by influenza B (Yamagata lineage) with an increase in influenza A (H3N2) and (H1N1)pdm09 during the second half of the epidemic (table 1 and figure 1B): in our hospital 100% influenza A was detected in 2016-2017 vs 36% influenza A in 2017-2018. Vaccine effectiveness was comparable in 2016-2017 and 2017-2018 (47% vs 44%, respectively).12,13 During the 2017-2018 influenza season, a high number of elderly people with pneumonia was registered nationally as compared to the previous year, as well as a sharp increase in mortality.14 Six percent more patients were seen at the ED in 2017-2018 compared to 2016-2017 (87 vs 92/day, respectively; p = 0.004, table 1). When compared with 2016-2017, a higher number of influenza tests was performed in the 2017-2018 season, with a higher proportion of influenza-positive results (32% vs 40%, respectively; p = 0.0003) in younger patients (76 vs 72 years, respectively; p = 0.0001, table 1).
Clinical performance of POCT
Overall rate of agreement between the Cobas Liat and Panther Fusion assay was 100% for influenza A and 98.3% for influenza B (Supplementary table 2). Reciprocal testing showed that the only two discrepancies (one NT swab tested positive, while the other NT swab from the same patient tested negative) were due to differences in influenza load between the first and second NT swab and/or low RNA load (cycle threshold value of 44.0). As expected, turnaround times were sharply decreased compared to the 2016-2017 epidemic: time from ED presentation to sample collection (194 in 2016-2017 vs 47 min in 2017-2018, p < 0.0001) and time from sample collection to result (1094 in 2016-2017 vs 62 min in 2017-2018, respectively; p < 0.0001) were both significantly shorter (table 1). ED patient-time was increased for all patients in 2017-2018 compared to 2016-2017 (2.65 vs 2.73 hours, respectively; p = 0.0005), but was lower for influenza-positive patients (3.83 vs 3.63 hours, respectively; p = 0.028).
Patient treatment and hospital flow
A significantly lower percentage of influenza-positive patients was admitted from the ED to the hospital when comparing 2016-2017 to 2017-2018 (91% vs 73%, respectively; p < 0.0001; including influenza A-positive patients only, 91% vs 70%, respectively, table 1). This was also true for patients who were tested influenza-negative (93% vs 80%, respectively; p < 0.0001) but not for all ED patients (39% vs 38%, respectively; p = 0.23). Compared to 2016-2017, length of stay of influenza-positive patients was shorter in 2017-2018 (5.86 vs 4.61 days, respectively; p < 0.0001; for influenza A-positive patients only, 5.86 vs 3.91 days, respectively; p < 0.0001). A significantly longer length of stay was observed for all admitted influenzapositive patients in 2016-2017 compared to patients initially on the influenza ward in 2017-2018 (5.86 vs 4.43 days, respectively; p < 0.0001), but no difference was observed between patients on the influenza ward and those on other wards in 2017-2018 (4.43 vs 5.09 days, respectively; p = 0.32). During weeks 7-10 of 2017-2018, the 15-bed capacity of the influenza ward was insufficient (figure 1C). The average time from hospital admittance to test result was 16.15 hours in 2016-2017. Since 635 patients were tested negative for influenza and admitted subsequently in 2017-2018, 427 patient-days of unnecessary isolation were prevented (636*16.15 = 10,255 hours). The POCT result was known, on average, 49 minutes before admission, due to several aspects of the clinical pathway, including 1) faster sample collection at the ED, 2) shorter test turnaround time, and 3) no extra time for communicating the result from the laboratory to the clinician.
Antibiotic use was significantly reduced in 2017-2018 compared to 2016-2017, at a similar rate for both influenzapositive and -negative patients (table 1). Oseltamivir use however was significantly lower for admitted influenzanegative patients (5% in 2016-2017 vs 0.6% in 2017-2018, p < 0.0001), most of which was accounted for by oseltamivir that was started in the ED (4% in 2016-2017 vs 0.4% in 2017-2018; p < 0.0001). For influenza-positive patients, no significant difference in oseltamivir use was observed between the 2016-2017 and 2017-2018 seasons (9% vs 11%, respectively; p = 0.4). The rate of bacterial or fungal superinfection (defined as positive culture of respiratory tract material or positive pneumococcal antigen test) was not significantly different either in admitted influenza patients during the 2016-2017 and 2017-2018 seasons (10% vs 14%, respectively; p = 0.17; for influenza A-positive patients only, 10% vs 12%, p = 0.55).
The vaccination rate of employees on the influenza ward was 74%, whereas the same department (then Geriatrics) in 2016 had a vaccination rate of 47%. During weekly gatherings, all disciplines involved declared to endorse the implementation of the clinical pathway.
Costs
Estimated costs of the laboratory aspects of the clinical pathway are 73,822 euros (personnel 43,822; technical 30,000) and estimated costs of the clinical aspects are 27,367 euros (ED 3,367; other staff 24,000), leading to a total of 101,189 euros (for details see Supplementary Materials). Estimated savings due to shorter length of stay are 257,644 euros, those due to decreased hospital admissions 234,125 euros, and savings due to fewer unnecessary isolations 6,450 euros, leading to a total estimated saving of 498,219 euros. Subtracting the costs from the savings gives an estimated saving of 397,030 euros for influenza season 2017-2018.
DISCUSSION
Overall, our hospital had a positive experience with the implementation of the clinical pathway for influenza. Test performance was good and turnaround times significantly reduced. Comparing the epidemics of 2016-2017 and 2017-2018, ED patient-time was slightly decreased for influenza-positive patients, even while an increase for all ED patients was observed. A lower percentage of influenza-positive patients was admitted, which was also true for patients with a negative influenza test, but not for all ED patients. Increased ED discharge rates could be due to faster influenza test results, available for a larger proportion of presenting patients; the prevailing viruses are unlikely to be responsible, since the 2017-2018 season appeared to be more severe with a higher incidence of pneumonia compared to the previous year and higher mortality was observed. Length of stay of both influenza A and B-positive patients was shorter compared to the previous season, which could be related to more efficient clinical decision making and the short-stay nature of the influenza ward. A strong reduction in unnecessary isolation was accomplished, which is an important factor in improving hospital patient flow.
The reductions in admissions, length of stay, and unnecessary isolation combined were roughly estimated to have saved 397,030 euros. To gain better insight into the costs and savings from a healthcare provider perspective, a more sophisticated analysis including costs associated with additional or avoided diagnostics is required. We do not expect this to strongly alter the result: the number of additional tests related to respiratory tract infection was not significantly different in 2016-2017 compared to 2017-2018 (sputum culture in 31% vs 30% of patients and Legionella and Streptococcus pneumoniae antigen tests both in 4% vs 4% of patients, respectively). Our data do not indicate that a more severely ill selection of influenza-positive patients was admitted in 2017-2018 compared to 2016-2017: The percentage of superinfections and ICU admissions was not different; a lower percentage of patients received antibiotics and the length of stay was shorter.
In setting up a clinical pathway for influenza, several aspects should be considered. The single-sample capacity of the POCT can be a disadvantage if many patients with respiratory disease present at once. It proved useful to transfer the second POCT from the microbiology reception desk to the ED during peak moments. Near the end of the epidemic, it was considered better to not include fever criterium into the case definition, but only acute respiratory tract infection, since cases might be missed.
Retrospectively, the 15-bed influenza ward should have been twice as large during the peak season. The estimation of the required capacity was calculated based on previous season’ s numbers: the discrepancy could be due to both a different algorithm for and way of testing, and the epidemic in general being more severe compared to the previous year.
Few studies have been published on PCR-based influenza POCT in daily practice. Gibson et al. found 99.6% and 99.3% percent positive agreement for the Cobas Liat Influenza test compared to another PCR-based test in 1361 nasopharyngeal swabs (both primary care and ED).5 Trabattoni et al.7 used the Alere i Influenza A&B POCT in 132 ED patients and found reduced hospitalization rates and a reduced number of additional diagnostic tests compared with routine testing, but no differences in prescription of antibiotics. In contrast with the modest reduction in ED patient-time we observed, this study found a strong reduction from approximately six hours to four hours; however, in our study ED patient-time was already four hours before implementation of POCT. Brendish et al.4 performed the only randomized controlled trial so far, using the FilmArray Respiratory Panel, which includes 17 viruses and 3 bacteria. They found a reduced length of hospital stay for patients assigned to POCT (n = 362, mean 5.7 days) versus routine care (n = 358, mean 6.8 days). This observation was most pronounced among patients with exacerbations of airway disease, in whom also a significant reduction of antibiotic duration was reported. Patient time in ED was not reported; mean POCT turnaround time was 2.3 hours. Influenza POCT was shown to provide overall cost savings due to changes in physician decision making.15,16
To the best of our knowledge, this is one of the first reports on implementation of a clinical pathway for influenza, focusing not only on the POCT itself but on all aspects involved in clinical care for influenza patients during an epidemic. This study has a number of limitations. Due to its retrospective nature, no causal relationships can be inferred. Circulating viruses and severity of both epidemics could affect many of the reported values. The slightly younger age of influenza-positive patients could be a confounder. While in 2017-2018, the case definition for testing was strictly adhered to, it is possible that in 2016-2017 some influenza-positive admitted patients remained undetected, rendering our comparison incomplete.
Overall, implementation of the clinical pathway for influenza patients proved a success in terms of practical and logistical execution in a multidisciplinary setting. Based on our results, the clinical pathway has likely improved patient flow, possibly leading to a lower percentage of admissions and shorter length of stay. Moreover, it has likely led to a significant reduction in costs. We would recommend hospitals with settings similar to ours to explore possibilities in improving patient flow during the influenza epidemic. Our data may not be applicable to, for example academic hospitals, which have a very different type of patient population, when commissioning a different POCT, when plenty of single rooms are available, or when the laboratory is open 24/7. Further research is needed to dissect the full impact of implementing an influenza clinical pathway, but the first results are promising.
ACKNOWLEDGEMENTS
The authors would like to thank all involved JBH employees for all the effort they put into this project, especially Kathelijn Geraats, Marcel Sterks, Jeroen Schellekens, Monic Janssen, Jaap Kroon, Karin Zeegers (key users of the Cobas Liat system); John de Laat, Mieke van Bergen, Natasja van Duijvendijk, Karin Oostelbos (Head of Unit of the Emergency Department, Influenza Ward and Microbiology Department, respectively), Peter Wever (local responsible investigator), Saskia Walbeek, Eline Lohstroh (quality officers), Eric Smits, Theo van Well (department managers), Marleen Henskens and colleagues of the Infection Control team, the Management information team, Jens Verhagen and colleagues of the communication department, all planning nurses under supervision of Yvonne Kouwenberg and all the nurses, doctors, and laboratory personnel who contributed to making this influenza season a success in our hospital.
PREVIOUS PRESENTATION OF DATA
Part of the data from this paper was presented as a poster at the Scientific Spring Meeting of the Dutch Society for Microbiology (NVMM/KNVM), Papendal, The Netherlands, March 2018. In addition, part of the data was published as a short, non-scientific report in Dutch in Medisch Contact, section ‘Beleid’, October 31st, 2018.
DISCLOSURES All authors declare no conflicts of interest. No funding or financial support was received.
* For the Supplementary Materials, please contact the corresponding author.
REFERENCES