

KEYWORDS
Expression, MMP-2 and -9, secretion, SLE, TIMP-1 and -2
INTRODUCTION
Systemic Lupus Erythematosus (SLE) is a systemic autoimmune disease characterised by altered autoantibody formations against various autoantigens resulting in direct cellular and immune complex-mediated tissue damage.1,2 Multiple studies have analysed the role of matrix metalloproetinases (MMPs) and their endogenous inhibitors (TIMPs) in the pathogenesis of autoimmune disorders such as Wegener’s granulomatosis, multiple sclerosis, scleroderma, and Sjogren’s syndrome.3-6
MMP-9, MMP-2, and their tissue inhibitors TIMP-1 and TIMP-2 have been implicated and linked to inflammation in autoimmune disorders, in particular, SLE.7,8 Earlier studies have shown conflicting results. The studies done in China and Israel reported higher levels of both MMP-9 and MMP-2,8-10 where as studies carried out in Poland and Iran reported a higher MMP-2 level but lower MMP-9 levels in the serum of SLE patients.10-13 A recent meta-analysis of levels of serum MMP-9 in SLE based on more than 700 patients with SLE and healthy controls did not find any significant association. However it did find that the sample size (n ≥ 60) group had lower MMP-9 levels as compared to controls.14
MMP-9 and MMP-2, also known as gelatinase B and gelatinase A, cleave denatured collagens (gelatins) and type IV collagen, the major component of basement membranes. This results in the migration of various inflammatory players including lymphocytes and other leucoocytes, which enter and leave the blood circulation. In addition, MMP-9 cleaves some proteins leading to remnant epitopes that can generate autoimmunity.15 Recently, an abnormal regulation of neutrophil extracellular traps (NETs) has been reported in SLE. They are released by activated neutrophils by a process called ‘NET formation’.16 We hypothesised that the abnormal expression and secretion in neutrophils and lymphocytes of MMP-9 and MMP-2 may lead to disease exacerbation and may play a role in perpetuation of autoimmunity in SLE. It may act as a potential marker for SLE disease progression.
MATERIALS AND METHODS
A total of 41 diagnosed SLE cases (38 females and 3 males) fulfilling the revised American College of Rheumatology (ACR) classification criteria for SLE (1997)17 were recruited along with 50 age and sex matched healthy controls (47 females and 3 males) without any concurrent infections between November 2013 to December 2015 (three years and one month). The study was approved by the Institutional Ethics Committee (IEC) and written consent was obtained from each patient and controls recruited. Anti-nuclear antibodies (ANA) and anti-dsDNA were detected by indirect immunofluorescence (IIF) method and high-sensitivity C-reactive protein (hs-CRP) levels were measured by nephelometer analysis (BN Prospec, Germany).
Peripheral blood mononuclear cell (PBMC) culture
PBMCs were isolated from fresh peripheral blood by Histopaque-1077 (Sigma-Aldrich, USA). After purification, one part of the 1 x 106 PBMCs/ml was directly used for RNA isolation, and another part was cultured at 37 °C and 5% CO2 under serum-free conditions in RPMI 1640 (Sigma Chemical, USA) supplemented with Nutridoma-SP (Boehringer Mannheim, Mannhein Germany) in the presence of antiobioic-antimycotic solution (HiMedia, India); all the PBMC cell populations (active, inactive, and controls) were activated with 10 μg/ml of phytohemagglutinin (PHA). After 24 hours (hrs), conditioned media was harvested and culture supernatants stored at -20 °C until use.
ELISA Analysis of serum and conditioned media
Matrix metalloproteinases (MMP-2 and MMP-9) and tissue inhibitors of MMPs (TIMP-1 and TIMP-2) were analysed in SLE cases (n = 41) and controls (n = 50) in their respective serum and PBMC culture supernatant conditioned media samples using the RayBio human sandwich enzyme-linked immunosorbent assay system (RayBiotech, Norcross, Georgia, USA) as per the manufacturer’s protocol and instructions.
mRNA expression analysis of MMPs and TIMPs
Fresh whole blood (WB) (500 µl) and 1 x 106 PBMCs/ml (500 µl) were used for RNA isolation by the RiboPure RNA Purification Kit (Ambion, Life Technologies) for 41 samples of SLE patients and 50 controls and convereted to cDNA. The mRNA expression was studied using Taq Man Hydrolysis FAM-MGB labelled probes MMP-2 (Hs01548727_m1), MMP-9 (Hs00234579_m1), TIMP-1 (Hs00171558_m1), TIMP-2 (Hs00234278_m1), and18s (Hs99999901_s1) was used as a reference gene. Data was acquired using the Step-one software version 2.2.2. Data expression levels were recorded as Cq. The mean Cq values of the duplicate reactions were used in further analysis.
Intracellular Assessment of MMP-9 and MMP-2 by flow cytometry
Fourty-one SLE cases and 50 controls were included for whole blood flow cytometry work. The permeabilisation protocol was used to detect the intracellular as well as the surface protein antigen in the whole blood. Cells were stained with the following primary monoclonal antibodies: mAb anti-MMP-9 (EP 1255Y) and mAB anti-MMP-2 antibody (EP 1183Y; Novus Biologicals, Littleton, USA). A 1:100 diluted secondary conjugated goat anti rabbit IgG H & L (AlexaFl 488; Abcam, Cambridge, USA) antibody was then added. The results were calculated dividing signal to noise (noise: the reading obtained from the secondary tube that was run with each sample, which contained only secondary antibody and sample) and thus acted as an adjustment for background staining. Cells were analysed using a BD FACSAria (BD, San Jose, CA, USA) flow cytometer and the obtained data were analysed and visualised using the BD FACSDiva (BD, USA) software. The output for the intracellular MMP-2 and MMP-9 was given in terms of mean fluorescence intensity (MFI) and percentage positivity(%).
Statistics
GraphPad Prism 6.0 was used for statistical analyses. Data was presented as mean ± SD. The statistical significance between two groups was tested by using Mann-Whitney U-test. Wherever more than two groups were analysed, ANOVA analysis was performed and the results represented in Median-IQR. Spearmans’s rank correlation was used for correlation analyses. A P-value < 0.05 was considered statistically significant.
RESULTS
The mean age of SLE patients at the time of evaluation was 28.34 ± 9.25 years. The mean disease duration was 2.20 ± 2.1 years. Severity of the disease was assessed by calculating the SLE Disease Activity Index (SLEDAI).18 The mean SLEDAI score for patients was found to be 13.0 ± 5.2. Based on the SLEDAI score, patients were categorised into active (SLEDAI ≥ 10) and inactive (SLEDAI < 10) groups. Accordingly, there were 22 patients (21 females and 1 male) in the active group and 19 patients (17 females and 2 males) in the inactive group. In active cases, 12 (54%) cases had lupus nehritis (LN). Of the 41 disseminated lupus erythematosus patients, 39 patients (95.1%) were on corticosteroids, 33 patients (80.48%) were on anti-inflammatory drug (HCQ), 5 patients (12.1%) were on cyclophosphamide, and 4 patients (9.7%) were on alternative biologics.
Serum levels of MMPs and TIMPs
We analysed the circulating protein concentration of MMPs and TIMPs in serum. As shown in figure 1A, circulating MMP-2 concentrations and TIMP-1 secretion were highest in cases with active disease compared to inactive disease (p = 0.0003, p < 0.0001, respectively). In contrast, circulating MMP-9 concentrations were found to be lowest in active cases as compared to inactive cases and healthy controls (p < 0.0001). TIMP-2 secretion was reported to be highest in inactive cases. This result indicated an alteration of MMP/TIMP secretion profiles in the SLE disease activity state.
Gene expression of MMPs and TIMPs
We analysed the mRNA expression in WB as well as fresh PBMCs. WB MMP-2 gene expression was highest in the active cases as compared to inactive cases and controls (p < 0.0001) (figure 1B). These results are in accordance with the protein secretion pattern seen in serum. Similarly the whole blood mRNA expression of TIMP-2 was found to be highest in inactive cases as compared to active cases and controls with no significance. The results were in concordance with the protein secretion of TIMP-2 seen in serum. The MMP-9 gene expression was highest in inactive cases; the MMP-9 gene expression of active cases was also higher as compared to controls, which was in contrast to the secretion profile in serum. TIMP-1 gene expression was highest in inactive cases. This led us to analyse the PBMCs gene expression profile. In PBMCs, the expression of MMP-2 was 12-fold higher in active cases and 6-fold higher in inactive cases compared to controls (figure 1C). Similar observation was reported for TIMP-1 gene expression in active cases [Median - interquartile range (IQR)]; [Relative Quantification (RQ) = 14.91 (14.56 – 15.49)], and TIMP-2 expression in inactive cases [RQ = 3.41 (3.0 – 3.78)] as compared to controls [RQ = 1.13 (1.05 – 1.29)].
Secretional status of MMPs and TIMPs in PBMC-cultured cells
We measured secreted MMPs and TIMPs levels in culture supernatants (CS) after 24 hours (figure 1D). Data showed that, even in the absence of any external stimulation, PBMCs spontaneously released significantly higher quantities of MMP-2 in active cases [15.97 ng/ml (15.51 – 16.51)] as compared to inactive cases [10.14 ng/ml (9.36 – 10.55)] which in turn were higher than healthy controls [7.37 ng/ml (7.09 – 7.98)]. The secretional levels of MMP-9 were significantly reduced in active cases [7.36 ng/ml (6.97 – 7.94)] as compared to inactive cases [14.08 ng/ml (13.61 – 14.44)]. For endogenous inhibitors the secretion of TIMP-1 was higher in active cases [11.93 ng/ml (11.40 – 12.86)] as compared to inactive cases [5.49 ng/ml (4.7 – 6.22)].
Intracellular staining of MMP-2 and MMP-9
The altered expression pattern in WB and PBMCs led us to analyse the presence of MMP-2 and MMP-9 in circulating neutrophils and lymphoctyes by WB flow cytometry (figure 2A). Among SLE patients, MFI values of MMP-9 were significantly higher in neutrophils (6.11 ± 0.12) as compared to healthy controls (2.96 ± 0.10, p < 0.0001), but no difference was observed in the lymphocytes (p = 0.32); the MMP-2 values were higher in lymphocytes (5.51 ± 0.72) as compared to healthy controls (1.65 ± 0.12, p < 0.0001). A similar observation was reported in neutrophils (p < 0.0001) (figure 2B). Among SLE patients, the majority of the lymophocytes showed positivity for MMP-2. In neutrophils, the positivity for MMP-2 was less in SLE cases than in controls. Among the control group, majority of neutrophils showed positivity for MMP-9 (79.98 ± 3.82%) as compared to SLE cases (70.67 ± 3.21%). In lymphocytes, the positivity for MMP-9 was decreased in SLE cases (30.36 ± 4.71%) as compared to controls (48.72 ± 4.57%) (figure 2C).
Gelatinase/Inhibitor ratios
To calculate the relative inhibition of the two MMPs, we calculated the ratio between MMPs and TIMPs (table 1). The MMP-2/TIMP-1 ratio showed a consistent pattern of decrease in ratio for active cases as compared to inactive cases for serum secretion. Similar results were obtained for the MMP-9 /TIMP-1 ratio. The ratio of MMP-2/ TIMP-2 showed a consistent pattern of increase in active cases as compared to inactive cases which in turn, were significantly higher than controls for serum secretion. The MMP-9/TIMP-2 ratio showed a pattern of increase in controls as compared to active and inactive cases for serum secretion. 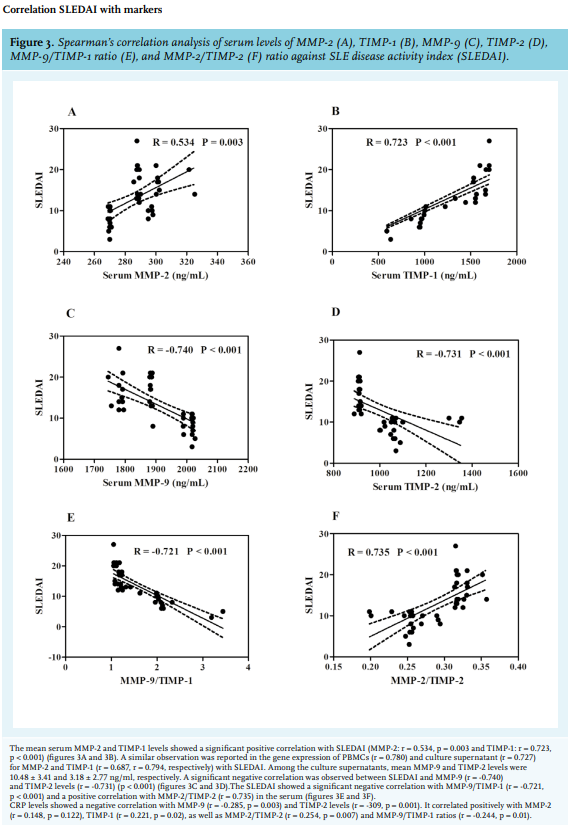
Recent evidence suggests that MMPs are involved in many types of kidney disease.19 A few studies have been carried out in SLE patients to understand the role of MMPs and TIMPs in the pathogenesis of SLE. Studies carried out in the Chinese SLE cohort by Jiang et al. reported significantly higher levels of serum MMP-2 in advanced stages of the disease.8 A similar observation was reported by Bahermand et al. in Iranian SLE cases.12 In our study, the serum concentrations of MMP-2 were found to be highest in active cases as compared to inactive SLE cases. The results were in concordance with a study conducted in Greece in kidney biopsies of lupus nephritis patients, which showed a marked increase in expression of MMP-2 and significantly correlated with the higher activity index as compared to absent or weak staining (p < 0.001).20
A study carried out in Polish population by Robak et al. in 41 SLE cases and 20 healthy controls reported a decrease in serum levels of MMP-9 in active SLE cases as compared to controls (p = 0.03).11 Similarly, in accordance with our results, a study done by Liu et al. in 31 SLE cases in PBMC cell culture supernatants reported a decrease in MMP-9 levels in relapsed cases (5.46 ± 4.27 ng/ml) as compared to the patients in remission (12.98 ± 8.07 ng/ ml).21 A study done in Iranian populations by Bahrehmand et al. showed a significant decrease in serum levels of SLE cases as compared to controls (p < 0.001) which are in line with our study.13 Similar results were obtained in our study, showing lowest MMP-9 serum concentrations in active cases than inactive cases. Serum concentrations of TIMP-1 were higher in active cases as compared to inactive cases. TIMP-1 levels were significantly lower in inactive cases as compared to controls, indicating an alteration in the serum secretion of TIMP-1 as compared to its substrate MMP-9.
A study by Matache C et al. also reported a decrease in the PBMC secretion of TIMP-1, which was not sufficient to inhibit the MMP-9 activity.7 Further, we revealed that TIMP-2 levels were higher in SLE patients as compared to controls; a similar observation was reported by Jiang et al.in Chinese population.8 The mared alterations were observed in the gene expressions of MMPs and TIMPs in WB and PBMCs among SLE patients. MMP-2 and TIMP-2 showed a similar mRNA expression pattern in WB, Fresh (F)-PBMCs, and PBMC culture supernatants as seen in serum levels. A study carried out by Matache et al. reported a consistent pattern of increase in mRNA expression of MMP-9 in F-PBMCs and protein secretion in culture supernatants in inactive cases as compared to active cases.7 Similar findings were observed in our study which extended further to WB mRNA expression and a profile similar to secretion of MMP-9 in serum of inactive cases.
The studies conducted by Jonssonet al. which compared mRNA expression of MMP-9 and TIMP-1 in PBMCs with circulating protein concentration failed to find any correlations.22 This disparity was further reported by Jonsson et al. in myocardial infarction patients23 and Lichtinghagen et al. in chronic hepatitis C patients,24 in which they assessed mRNA expression of MMP-9 and its inhibitors in cultured cells but found no significant correlation. Similar results were obtained in our study, which are suggestive of the fact that the MMP-9 and TIMP-1 mRNA is being converted into proteins.
Also, MMP-9 is mainly found in the intracellular tertiary granules of neutrophils.25 Due to the altered expression and secretion pattern of PBMCs and WB in SLE cases, we decided to analyse this difference in the intracellular levels in neutrophils and lymphocytes. Results confirmed that neutrophils were the predominant source of MMP-9, and lymphocytes as the predominant source of MMP-2 in SLE cases. Although TIMP-1 is an endogenous inhibitor of MMP-9, studies have shown that TIMP-2 has been more effective in inhibiting MMP-9.26 A study carried out by Jonsson et al. reported contrasting findings to our study in post mycardial infarction patients, in which they reported a significant increase in MMP-9 mRNA expression in PBMCs, which showed a positive correlation with TIMP-1 and TIMP-2, and between TIMP-1 and TIMP-2 at mRNA and protein levels.23 We found a significant positive correlation between MMP-9 and TIMP-2 in fresh PBMC mRNA and culture supernatants (CS) which were similar to the results obtained by Jonsson et al.22 We also reported a positive correlation between WB mRNA and CS levels for TIMP-1 and TIMP-2.
MMP-2 and TIMP-2 negatively correlated at the serum level and positively at the CS level; an explanation for this would be that in our study, MMP-2 has been mainly found to be expressed and secreted in PBMCs of SLE cases. This study signifies a marked alteration in matrix degradation resulting in enhanced matrix deposition in SLE cases, hence we calculated the ratios between MMPs and TIMPs. Low MMP-2/TIMP-1 and MMP-9/TIMP-2 has been reported in previous studies. The study carried out by Lichtinghagen et al. in peripheral blood cells of hepatitis C induced cirrhosis (Ci) patients reported a lower ratio of MMP-2/TIMP-1 and MMP-9/TIMP-2 as compared to controls.24 Also a study carried out in chronic hepatitis C patients before therapy, according to alanine transaminase (ALT) responses to interferon, reported a significantly lower MMP-2/TIMP-2 ratio in patients with sustained response than in those with transient response and no response. The authors therefore concluded that the ratio of serum MMP-2/TIMP-1 levels may act as a new predictor for interferon response in patients with chronic hepatitis C patients.27 Our study also showed a consistent reduction in the pattern of MMP-2/TIMP-1 and MMP-9/ TIMP-1 in active cases at all levels, which is suggestive of the increase in TIMP-1 expression and secretion. In the MMP-2/TIMP-2 ratio, a consistent pattern of decrease in the order of active, inactive, and controls conveys the fact that MMP-2 expression and secretion increases as the disease progresses, which is in agreement with previous studies.12
The studies carried out by Ahmad et al. in the Egyptian population 28 and Liu et al. in the Chinese population29 showed a significantly negative correlation of MMP-9 levels with the SLEDAI scores. A similar negative correlation of MMP-9 levels with the SLEDAI scores was observed in our study. Also, a study conducted by Cauwe B et al. in a gelatinase B/MMP-9-deficient mouse model (B6lpr/lprMMP-9- /- ) showed that the MMP-9 deficiency aggrevated lymphoproliferation-induced a lupus-like systemic autoimmune disease.30 The above findings were confirmed in a study conducted in LPR- /- MMP-9- /- double knockout mice that showed an increase in the level of immune-complex in the plasma and also local complement activation in spleen and kidney. It further showed that MMP-9 dissolved immune complexes from plasma of the lupus-prone mice and from blood samples of SLE patients.31
Also, a significant positive correlation of serum CRP levels with TIMP-1 levels (r = 0.221, p = 0.02) in our study are in accordance with the study carried out in Wegener’s granulomatosis patients by Bjerkeli V et al., which reported a significant positive correlation of TIMP-1 levels with CRP (r = 0.74, p = 0.004).3
MMPs have been shown to be modulators of inflammation and autoimmunity, especially SLE.19,25 In this study, we found a decrease in MMP-9 and an increase in MMP-2 as SLE disease progresses. We tried to analyse these alterations by studying their indigenous inhibitors TIMP-1and TIMP-2, respectively. To make a complete analysis, we studied the mRNA expression in WB and F-PBMC; secretion of proteins in serum and PBMC CS. Our findings support the theory by Robak et al. that lower MMP-9 concentartions in serum may be suggestive of the fact that in SLE, MMP-9 is transported from blood to the lupoid tissues, especially the blood vessels of active SLE patients. We further extend the theory to neutrophil extracellular traps (NETs), which have been shown to be abnormally regulated in SLE.15,32 There, clearance is impaired, leading to abnormal alteration in MMP-9 secretion and expression, which has been proven for the first time in our study in intracellular levels of neutrophils and lymphocytes of WB for MMP-2 and MMP-9. Our study suggests a significant increase of MMP-9 in neutrophils and MMP-2 in lymphocytes in SLE cases. Therefore, an alteration of MMP/TIMP ratios suggests its importance in the pathogensis of SLE disease progression.
In summary, our study suggests that the gene expression and serum proteins of MMP-9 and MMP-2, and their natural inhibitors TIMP-1 and TIMP-2 can be used as a potential biomarkers for SLE pathogensis and they correlate with the SLE disease activity. Therefore, an alteration of MMP/TIMP ratios suggests its importance in the prognosis of SLE disease progression.
ACKNOWLEDGEMENTS
We thank the Indian Council of Medical Research (ICMR), New Delhi, India and University of Mumbai for their support. We would like to thank Dr. Swati Garg and Mrs. Aparna Dalvi for their technical advice in flow cytometry.
DISCLOSURES
All authors declare no conflicts of interest. No funding or financial support was received.
REFERFENCES