

KEY WORDS
Allergic diseases, allergic diseases and cancer, atopy, cancer, malignancy
INTRODUCTION
Immunoglobulin E (IgE)-mediated allergic diseases (hereon called allergies) are frequently reported, especially in developed countries, and result in high morbidity and high costs for healthcare systems.1 The most commonly reported allergies are atopic diseases (allergic rhinitis, asthma and eczema) and food allergies. The diagnostics and treatment options for patients with allergies have improved significantly in the past decades. Although still controversial, the hygiene hypothesis proposes a decrease in infectious disease in early childhood as the cause of high incidence of allergies and asthma in developed countries.2 The lack of early infections leads to the stimulation of a T-helper 2 (Th-2) cell-mediated immune response favoring allergic diseases. The genetic susceptibility of the host however, may also play a key role in developing atopic symptoms.3
Previous studies have highlighted the potential inverse association between allergies and cancer.4-6 Currently, the association between allergy and oncology is of high interest and the European Academy of Allergy and Clinical Immunology (EAACI) has established a Task Force to better understand basic immune responses in both fields.7 Patients with allergic diseases may develop a state of enhanced immune surveillance leading to fewer occurrences of malignancies such as glioma and pancreatic cancer.8,9 The purpose of this study was to give an overview of the association between allergic diseases and different types of cancers by performing a systematic review of the literature.
METHODS
A systematic literature search was performed to include all articles that addressed an association between allergy and cancer. This systematic review was performed and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.
Outcomes were structured by cancer category. The number of studies reporting a positive, negative or no association was noted. For each category the lowest and highest values of the most frequent risk estimates, such as the relative risk (RR) odds ratio (OR), hazard ratio (HR) and the standardized incidence ratio (SIR) were reported.
Data source
Studies on the association between allergy and cancer were conducted from the following online databases: Embase, Ovid Medline, Web of Science, Cochrane Library and Google Scholar. The last search was run on June 2nd, 2017. No filters for date or language were used in the search strategy (see the additional Appendix for the full search strategies).
Study selection
The titles and abstracts of all studies were reviewed after extracting duplicates. The studies were evaluated using the following criteria for inclusion: articles in English or English translation, original studies focusing on the relationship between allergy and cancer. Studies focusing on serological parameters such as serum IgE and malignancy, in vivo and in vitro animal studies, review articles and meta-analysis were excluded. Three reviewers (AFK, LW, RO) independently performed a review of the full text and could reach consensus on the relevance for inclusion of each article.
Data visualization
In order to visualize the various risk estimates, we used the R Statistical Software to plot forest plots for all included studies and plotted separate forest plots for the different cancer subgroups. We made forest plots for all study outcomes reported in relative risk (RR), OR, HR and SIR. Studies that reported other outcomes, such as the standardized mortality ratio, are not plotted in the forest plots; neither are studies that did not report 95% confidence intervals. No overall estimates were calculated, as the use of different methods to express risk estimates did not allow us to pool the studies. For the same reason, the degree of heterogeneity of studies could not be calculated.
RESULTS
Of a total of 5868 articles identified by the search, 312 articles publishing an association between allergy and cancer were eligible (Supplementary figure 1). After screening, we further narrowed down our selection to 145 articles that reported an association between allergies and malignancies. The main outcomes of this study are shown in table 1, table 2 (at the end of this article) and figure 1, Forest plots of the association between allergic diseases and cancer types.
Brain cancer
In total, we identified 19 studies describing an association between allergic diseases and brain cancer.
Positive association with brain cancer: no study demonstrated a positive association between allergic diseases and a brain tumor.
Negative association with brain cancer: We included 19 studies reporting the association between allergic disease and brain cancer. Of these, six studies showed no association between allergic diseases and brain cancer;10-16 12 studies demonstrated a consistent reduced risk of brain tumors in patients with allergic diseases (OR between 0.34 and 0.76);11,17-28 and one study identified no association between allergic disease and meningioma, but did show a negative association between allergic disease and glioma. No studies reported a positive association.
Of the 19 studies, 16 examined the association between allergic diseases and brain cancer in adults, while three studies examined this association in children and adolescents.
Conclusion: Allergic diseases are mostly associated with a reduced risk of brain tumors.
Breast cancer
In total, eight studies on the association between allergic diseases and breast cancer were included.
Positive association with breast cancer: only one out of eight studies identified a positive association between allergic diseases and breast cancer (OR 2.5).29
Negative association with breast cancer: a negative association between allergic diseases and breast cancer was found in two studies (OR 0.77 and 0.86).30,31 Hedderson et al. found a decreased risk (OR 0.77) of breast cancer in women aged 35 years or older having a history of allergic diseases.30 However, in the same study, no association was observed between breast cancer and allergic diseases in women younger than 35 years. The other five studies did not identify a positive or negative association between allergic diseases and breast cancer.32-36
Conclusion: In general, allergic diseases do not appear to influence the breast cancer risk in women.
Lymphatic and hematopoietic cancer
We included a total of 47 studies on the association between allergic diseases and lymphatic and hematopoietic cancers. Negative as well as positive associations between allergic diseases and lymphatic and hematopoietic cancers have been published. Of the 47 studies, 20 showed no association, 15 reported a negative association (range OR: 0.29 to 0.87) and eight demonstrated a positive association (range OR: 1.3 to 3.84); four studies presented different associations for different subgroups.
Positive association with lymphoma: A positive association between allergic diseases and lymphoma was demonstrated in four studies (range OR:1.4-3.84).34,37-39 A positive association between airborne allergies and development of hematological malignancies, in particular, mature B-cell lymphoma (HR: 1.47) was found in one study.38 In this study however, the risk of malignancy was increased in women with a history of allergies to airborne allergens of plants, grass or trees, but not in men. Another study showed overall increased risk for non-Hodgkin lymphoma (NHL) in patients with allergic diseases (OR: 1.4).37 The high risk was mostly associated with erythema and allergic alveolitis, rather than with airborne allergies, and black patients with allergies seemed to be at a higher risk of developing NHL than white patients.
Negative association with lymphoma: A total of 10 studies showed a protective, negative association between allergic diseases and lymphoma (range OR: 0.29-0.87).40-49 In the study of Becker et al., hay fever and asthma did not influence the risk of lymphoma, and the association between food allergies and lymphoma was negative (OR: 0.67).47 The only study investigating the association between allergic diseases and NHL in children demonstrated a negative association (OR: 0.50).48
In total, 14 studies however could not find any association between allergic diseases and lymphoma.33,35,39,47,50-59
Positive association with other hematological malignancies: Seven studies of other hematological malignancies including lymphatic or myeloid leukemia, multiple myeloma and myelodysplastic syndrome, demonstrated positive associations between allergic diseases and these malignancies (range OR: 1.3-3.5).34,60-65 In Linabery et al., no association was found between allergic diseases and hematological malignancies, but a clear association was noted between asthma and myelodysplastic syndrome (HR: 2.17).63 This study was however limited to post-menopausal women.
Negative association with other hematological malignancies: A total of seven studies demonstrated a negative association between other hematological malignancies and history of allergic diseases (range OR: 0.4-0.6).66-72
A total of 10 studies (out of 47) examined the association between hematological malignancies and allergic diseases in children. As mentioned earlier, one study studied the association between allergic diseases and NHL and found a protective, negative association (OR:0.5).48 Another study found a positive association between allergic diseases and acute lymphocytic leukemia (ALL) in children (OR 2.2).64 The other 10 studies investigated the association between allergic diseases and leukemia in children. Only two studies showed a positive association between allergic diseases and leukemia (OR 1.4-1.7 and OR 2.2 respectively).60,64 In Spector et al., the early onset atopy was associated with increased risk of ALL, while there was no association between late onset atopy and ALL in children.64 In five studies, no associations between leukemia and allergic diseases were found.64,72-75
Conclusion: Most of the studies demonstrated no association between allergic diseases and lymphatic and hematopoietic cancers. However, there are more studies showing a protective role of allergies than studies with a positive association between allergic diseases and lymphatic and hematopoietic cancers.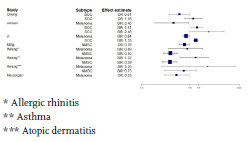
There is a limited number of studies on the association between skin cancers and allergic diseases available. We included seven studies.
Positive association with skins cancers: In two studies, a positive association between allergic diseases and skin cancer was described; SIR: 1.41 for basal cell carcinoma (BCC), SIR: 1.33 for squamous cell carcinoma (SCC).76,77
Negative association with skin cancers: A total of six studies described negative associations between allergic diseases and skin cancers (range SIR 0.39 to 0.84; range OR 0.53 to 0.78).76-82 In Cheng et al., the risk for developing BCC was decreased (OR: 0.61), while there was no association between allergic diseases and SCC.78 Two studies found a negative association between atopic dermatitis and non-melanoma scan cancers (NMSC) (OR 0.78 and SIR 0.40).79,80 Four out of five studies investigating the relationship between allergies and malignant melanoma observed a decreased risk for melanoma (range SIR: 0.46-0.84).76,77,81,82 In Hwang et al., the risk for developing malignant melanoma was unchanged in patients with allergic rhinitis, asthma and atopic dermatitis.80
Conclusion: Allergic diseases appear to reduce the risk for developing malignant melanoma and NMSC.
Colorectal cancers
We included 17 studies that evaluated the association between allergic diseases and colorectal cancers.
Positive association with colorectal cancers: Three studies described positive associations between allergic diseases and colorectal cancers (range SIR: 1.17-4.69).80,83,84 Vesterinen et al. found a positive association between asthma and colon carcinoma (SIR:1.17), but could not identify any association between asthma and rectal carcinoma.84 In Hwang et al., only a positive association was seen between asthma and colon carcinoma in women (SIR: 1.24).80
Negative association with colorectal cancer: A negative association between allergies and colon cancer was found in nine studies (range OR: 0.54-0.86).80,85-92 Prizment et al. studied the risk of colorectal cancer only in women and found a negative association (HR:0.74).86 Negri et al. described only a negative association between allergic diseases and rectum carcinoma, but no association between allergic diseases and colon carcinoma (OR: 0.64).90 In contrast La Vecchia et al. identified a negative association between allergic diseases and colon carcinoma, yet no association with rectal carcinoma (RR: 0.6).91 No associations were observed between allergic diseases and colorectal cancers in 11 studies.32,34-36,80,84,88,90,91,93,94
Conclusion: Overall, the risk of colorectal cancer is possibly reduced in patients with allergic diseases.
P
We identified 10 studies on the association between allergic diseases and pancreatic cancer.
Positive association with pancreatic cancer: No studies were published describing a positive association between allergic diseases and pancreatic cancer.
Negative association with pancreatic cancer: In total, seven studies reported a negative association between allergic diseases and pancreatic cancer (range OR: 0.43-0.77).9,95-100 In Olson et al., hay fever and allergies to animals were related to a reduced risk of pancreatic cancer, while asthma and other allergies did not appear to influence the risk of pancreatic cancer (OR 0.58).98 Three other studies did not observe an association between allergic diseases and pancreatic cancer.87,101,102
Conclusion: Overall, allergic diseases are associated with a reduced risk of pancreatic cancer.
Urogenital cancers
Among urogenital cancers, a total of 12 studies were identified reporting the association between allergic diseases and prostate cancer32-36,76,81,87,102-105 and seven studies described the association with female urogenital cancers.76,81,87,102,106-108
Positive association with urogenital cancers: In total, three studies demonstrated an increased risk of prostate cancer in patients with allergic diseases (range SIR: 1.18-1.64).76,102,105 Only one study showed a positive association between allergic diseases and cervix cancer (SIR 1.34),76 however, only the association between asthma and cancer was studied.
Negative association with urogenital cancers: Only one study showed a negative association between allergic diseases and prostate cancer (SMR 72).81 In eight studies, no significant association was described between allergic diseases and prostate cancer.32-36,87,103,104 Three studies on uterine leiomyomas, squamous cell cervical cancer, cervix and ovarian cancer showed reduced risks of these cancers in women with allergic diseases.81,106,107 In Kallen et al. however, only the association between asthma and cervix and ovarian cancer was studied.81 Four other studies looking at the association between allergic diseases and cervical cancer and ovarian cancer observed no changes in the risk of developing these cancers in women.76,87,102,108 Ji et al. however, despite observing no general association between allergic diseases and ovarian cancer, did see a positive association between asthma and cervix cancer (SIR 1.34).76
Conclusion: In general, there is possibly no association between allergic diseases and prostate cancer in men. Studies on the association between allergic diseases and female urogenital cancers are limited, but favor a protective role of allergies.
We included a total of 19 studies, which studied the relationship between allergic diseases and lung cancer.
Positive association with lung cancer: In 10 studies, increased risks of lung cancer in allergic diseases were observed (range HR: 1.26- 13).36,76,81,84,87,109-113 In Reynolds et al., the lung cancer risk was increased only in men with asthma.109 In all except for two studies,114,115 focusing only on examining the associations between asthma (and not other allergic disorders) and lung cancer, showed an increased risk of lung cancer. 33,35,36,76,81,84,87,109-113 In Turner et al., lung cancer mortality was increased in patients with asthma, but reduced in patients with hay fever only or patients with both asthma and hay fever (RR 1.11, 0.85 and 0.73, respectively).87 In another study, lung cancer incidence was increased in current smokers and former smokers with a history of asthma, but in patients with asthma who never smoked, the cancer risk was unchanged (HR 13, 4.0 and 0.6, respectively).113
Negative association with lung cancer: In five studies, negative associations between allergic diseases and lung cancer were found (OR 0.37-0.85).87,102,115-117 These studies usually examined the association between allergies (not only asthma) and lung cancer, and showed a reduced risk of developing lung cancer. The same applied to studies where, in general, no association was found between allergic diseases and lung cancer.32,33,35,113-115,118
Conclusion: Asthma is related with an increased risk of lung cancer, while atopic patients without asthma may be protected.
Other cancers
In total, we included 13 studies that studied the association between other cancers and allergic diseases.
Positive association with other cancers: In two studies, a positive association between allergic diseases and nasopharyngeal cancers was observed (OR 2.29 and HR: 2.33).119,120
Negative association with other cancers: Four studies found a negative association between allergic diseases and cancers, including head and neck cancers and rhabdomyosarcoma.121-124 However, no associations were noticed for most of other cancers including laryngeal cancer,125 neuroblastoma,119-126 biliary tract cancer,127 acoustic neuroma,128 oral squamous cell carcinoma129 and Kaposi’s sarcoma.130
Conclusion: Most of the studies published do not show a change of risk for developing cancers in patients with allergic diseases.
We investigated the risks of developing cancer in 12 studies.
Positive association with cancer: The risk of developing cancer was increased (OR 1.40) in only one study by McWorther.34 In this study, hives were associated with the strongest cancer risk and the strongest allergy association was with lymphatic-hematopoietic cancers.
Negative association with cancer: In total, five studies described a negative association between allergic diseases and cancer in general 131-135 Six other studies did not find an association between allergic diseases and cancer.35,36,80,136-138
Conclusion: Mixed results were noticed, but in general, allergic diseases may reduce the cancer risk.
DISCUSSION
In this systematic review, we described the association between allergic diseases and different types of malignancies. This review delivers a comprehensive overview of the risk of malignancies in patients with allergies.
In this study we demonstrate an inverse association between allergic diseases and most of the cancers. Allergic diseases appear to reduce the risk of brain cancer, pancreatic cancer, melanoma and possibly the risk of lymphatic and hematopoietic cancers, colorectal cancers, female urogenital cancers and cancer in general. The current available studies do not provide sufficient evidence for a protective role of allergic diseases in developing breast cancer and prostate cancer. Asthma appears to increase the risk of lung cancer, however, patients with atopic diseases without asthma possibly do not have an increased risk of lung cancer.
The question of interest is how allergies may cause immunosurveillance. Allergic immunity depends on Th2-cells, basophils, eosinophils, macrophages and the antibodies type IgG1 and IgE.139 Allergy is a consequence of improved and hyper-responsive immune system, which may possibly recognize dysregulated or damaged cells, including cancer cells, and may efficiently eradicate these cells (immunosurveillance hypothesis). Patients with allergic diseases have thus an adapted immune system, which may protect against cancers.140 The production of tumor-specific IgE alone, which has antitumor effects of dendritic cells, eosinophils, basophils and mast cells offer better tumor surveillance and reduced risk of cancers.7 Furthermore, it is suggested that allergic reactions in specific tissues may be able to remove mutagenic triggers before transformation to malignant cells occur (prophylaxis hypothesis).
Despite an inverse association between allergic diseases and cancer, asthma appears to be independently associated with increased risk of lung cancer, after adjustment for smoking habits. The patients with atopic constitution without asthma however, have a possibly reduced risk of lung cancers. Patients with asthma have mostly other subtypes of lung cancer than adenocarcinoma.141 Despite the protective role of allergies in cancers, patients with asthma are regularly characterized by airway inflammation, which possibly plays a crucial role in the pathogenesis of lung cancer. Chronic inflammatory conditions may promote development of cancer because of oxidative damage resulting in tumor suppressor gene mutations. Different studies have indeed demonstrated a relationship between chronic airway inflammation and lung cancer.142,143 Furthermore, recurrent treatment with local or systemic glucocorticoids may also lead to better tumor outcomes and increased cancer risk.6
A next interesting question addresses the role of allergen immunotherapy in the development of cancer. Studies have suggested that tumor microenvironment may favor switching to a tumor-specific IgG4, a less potent immunoglobulin, instead of IgG1 and IgE.144 IgG4 antibodies do not have sufficient immunostimulatory capacities, may block the cytotoxic activities of other antibodies and are correlated with shorter survival and disease progression.144,145 On the other hand, current data also suggest positive correlation between IgG4-related disease and enhanced cancer risk.146 In allergic patients undergoing allergen-specific immunotherapy, increased IgG4-specific antibodies have been observed and correlate with allergen tolerance.147 However, to date, no data are available on cancer incidence and mortality in patients being successfully desensitized.
The results of this study may be limited by studies relying on self-reported ascertainment of allergies, different methods of establishing the diagnosis of allergies, retrospective studies and not always adjusting for cofounders. The variety in methodology of the different studies did not permit us to calculate pooled estimates. Furthermore, by classifying all tumors in broad categories such as lung cancer, lymphatic and hematopoietic cancer, we do not consider possible differences between associations in subtypes of tumors. However, a substantial amount of evidence for the inverse association between allergic diseases and malignancies is reported. Exceptions are patients with asthma who have increased risk of lung cancer. Large prospective studies with validated measurement of allergies and data on potentially confounding factors are required for better understanding the association between allergy and oncology.
ACKNOWLEDGMENTS
We thank Wichor Bramer from the Erasmus University Medical Center for his expertise in biomedical information and systematic literature search.
DISCLOSURES
All authors declare no conflicts of interest. No funding or financial support was received.
APPENDIX
Search terms used in the medical database for the literature search in this systematic review on the association between allergy and cancer.
Embase.com (2694)
('allergy'/de OR 'atopy'/de OR 'rhinoconjunctivitis'/de OR 'allergic asthma'/de OR 'allergic rhinitis'/exp OR 'atopic dermatitis'/de OR 'food allergy'/exp OR (allerg* OR atopy OR atopic OR rhinoconjunctivit* OR (rhino NEXT/1 conjunctivit*) OR 'hay fever'):ab,ti) AND (oncology/de OR 'neoplasm'/de OR 'malignant neoplastic disease'/exp OR 'precancer and cancer-in-situ'/exp OR 'skin cancer'/ de OR 'cancer risk'/de OR 'cancer incidence'/de OR 'digestive system cancer'/exp OR 'breast cancer'/exp OR 'prostate cancer'/de OR 'bladder cancer'/de OR 'thyroid cancer'/de OR 'brain tumor'/exp OR 'lung cancer'/exp OR 'carcinogenicity'/de OR (oncolog* OR allergooncolog* OR neoplas* OR cancer* OR (tumo* NOT ('tumor necrosis factor')) OR malign* OR leukemi* OR leukaemi* OR glioma* OR glioblastoma* OR astrocytom* OR carcino* OR lymphoma* OR hodgkin OR myeloma OR meningioma* OR melonoma*):ab,ti) AND ('disease association'/de OR 'health hazard'/de OR 'hazard assessment'/de OR 'incidence'/de OR 'population risk'/de OR 'cancer risk'/de OR 'cancer incidence'/de OR 'odds ratio'/de OR risk/de OR 'neoplasm'/exp/dm_et OR 'risk factor'/de OR (((associat*s NEAR/6 (cancer* OR risk* OR disease* OR factor*)) OR ((risk*) NEAR/6 (cancer* OR disease* OR factor*)) OR hazard* OR incidence OR 'odds ratio' OR relationship* OR allergooncolog* OR (allergo NEXT/1 oncolog*)):ab,ti) AND ('observational study'/exp OR 'cohort analysis'/exp OR 'longitudinal study'/exp OR 'retrospective study'/ exp OR 'prospective study'/exp OR 'health survey'/de OR 'health care survey'/de OR 'epidemiological data'/ de OR 'case control study'/de OR 'cross-sectional study'/ de OR 'correlational study'/de OR 'population research'/ de OR 'family study'/de OR 'major clinical study'/de OR 'multicenter study'/de OR 'comparative study'/de OR 'follow up'/de OR 'clinical study'/de OR 'clinical article'/ de OR 'clinical trial'/exp OR 'randomization'/exp OR 'intervention study'/de OR 'open study'/de OR 'community trial'/de OR (((observation* OR epidemiolog* OR famil* OR comparativ* OR communit*) NEAR/6 (stud* OR data OR research)) OR cohort* OR longitudinal* OR retrospectiv* OR prospectiv* OR population* OR (national* NEAR/3 (stud* OR survey)) OR (health* NEAR/3 survey*) OR ((case OR cases OR match*) NEAR/3 control*) OR (cross NEXT/1 section*) OR correlation* OR multicenter* OR (multi* NEXT/1 center*) OR 'follow up' OR followup* OR clinical* OR trial OR random*):ab,ti) NOT ([Conference Abstract]/lim) AND [english]/lim NOT ([animals]/lim NOT [humans]/lim)
Medline ovid (1585)
("Hypersensitivity"/ OR exp "Rhinitis, Allergic"/ OR "Dermatitis, Atopic"/ OR exp "Food Hypersensitivity"/ OR (allerg* OR atopy OR atopic OR rhinoconjunctivit* OR (rhino ADJ conjunctivit*) OR "hay fever").ab,ti.) AND ("Medical Oncology"/ OR "Neoplasms"/ OR exp "Skin Neoplasms"/ OR exp "Digestive System Neoplasms"/ OR exp "Breast Neoplasms"/ OR exp "Prostatic Neoplasms"/ OR exp "Urinary Bladder Neoplasms"/ OR exp "Thyroid Neoplasms"/ OR exp "Brain Neoplasms"/ OR exp "Lung Neoplasms"/ OR (oncolog* OR allergooncolog* OR neoplas* OR cancer* OR (tumo* NOT ("tumor necrosis factor")) OR malign* OR leukemi* OR leukaemi* OR glioma* OR glioblastoma* OR astrocytom* OR carcino* OR lymphoma* OR hodgkin OR myeloma OR meningioma* OR melonoma*).ab,ti.) AND ("Association"/ OR "Incidence"/ OR "Odds Ratio"/ OR exp risk/ OR exp "Neoplasms"/et OR (((associat*) ADJ6 (cancer* OR risk* OR disease* OR factor*)) OR ((risk*) ADJ6 (cancer* OR disease* OR factor*)) OR hazard* OR incidence OR "odds ratio" OR relationship* OR allergooncolog* OR (allergo ADJ oncolog*)).ab,ti.) AND (exp "observational study"/ OR exp "Cohort Studies"/ OR exp "health surveys"/ OR "Health Care Surveys"/ OR "Epidemiological Monitoring"/ OR "Case-Control Studies"/ OR exp "Epidemiologic Studies"/ OR "multicenter study"/ OR "comparative study"/ OR exp "clinical study"/ OR "Random Allocation"/ OR (((observation* OR epidemiolog* OR famil* OR comparativ* OR communit*) ADJ6 (stud* OR data OR research)) OR cohort* OR longitudinal* OR retrospectiv* OR prospectiv* OR population* OR (national* ADJ3 (stud* OR survey)) OR (health* ADJ3 survey*) OR ((case OR cases OR match*) ADJ3 control*) OR (cross ADJ section*) OR correlation* OR multicenter* OR (multi* ADJ center*) OR "follow up" OR followup* OR clinical* OR trial OR random*).ab,ti.) AND english.la. NOT (exp animals/ NOT humans/)
Cochrane (150)
((allerg* OR atopy OR atopic OR rhinoconjunctivit* OR (rhino NEXT/1 conjunctivit*) OR 'hay fever'):ab,ti) AND ((oncolog* OR allergooncolog* OR neoplas* OR cancer* OR (tumo* NOT ('tumor necrosis factor')) OR malign* OR leukemi* OR leukaemi* OR glioma* OR glioblastoma* OR astrocytom* OR carcino* OR lymphoma* OR hodgkin OR myeloma OR meningioma* OR melonoma*):ab,ti) AND ((((associat*) NEAR/6 (cancer* OR risk* OR disease* OR factor*)) OR ((risk*) NEAR/6 (cancer* OR disease* OR factor*)) OR hazard* OR incidence OR 'odds ratio' OR relationship* OR allergooncolog* OR (allergo NEXT/1 oncolog*)):ab,ti)
Web of science (1239)
TS=(((allerg* OR atopy OR atopic OR rhinoconjunctivit* OR (rhino NEAR/1 conjunctivit*) OR "hay fever")) AND ((oncolog* OR allergooncolog* OR neoplas* OR cancer* OR (tumo* NOT ("tumor necrosis factor")) OR malign* OR leukemi* OR leukaemi* OR glioma* OR glioblastoma* OR astrocytom* OR carcino* OR lymphoma* OR hodgkin OR myeloma OR meningioma* OR melonoma*)) AND ((((associat*) NEAR/5 (cancer* OR risk* OR disease* OR factor*)) OR ((risk*) NEAR/5 (cancer* OR disease* OR factor*)) OR hazard* OR incidence OR "odds ratio" OR relationship* OR allergooncolog* OR (allergo NEAR/1 oncolog*))) AND ((((observation* OR epidemiolog* OR famil* OR comparativ* OR communit*) NEAR/5 (stud* OR data OR research)) OR cohort* OR longitudinal* OR retrospectiv* OR prospectiv* OR population* OR (national* NEAR/2 (stud* OR survey)) OR (health* NEAR/2 survey*) OR ((case OR cases OR match*) NEAR/2 control*) OR (cross NEAR/1 section*) OR correlation* OR multicenter* OR (multi* NEAR/1 center*) OR "follow up" OR followup* OR clinical* OR trial OR random*))) AND DT=(article) AND LA=(english)
Google scholar (200)
Allergy/allergies/allergic/atopy/atopic/rhinoconjunctivitis/ “hay fever” oncology/neoplasms/cancer/malignant/malignancies association/risk/ hazard/incidence/“odds ratio”/relationship
REFERENCES