

KEYWORDS
Type 2 diabetes, cardiorespiratory fitness, muscle strength, resting metabolic rate, body fat, lean body mass
INTRODUCTION
Type 2 diabetes mellitus has reached epidemic proportions worldwide, and is associated with obesity, and metabolic and cardiovascular disease. In the treatment of diabetes mellitus, reducing the risk of severe complications with irreversible organ damage is essential over the long-term.1 A multidisciplinary approach is also vital for health care professionals in the management of type 2 diabetes. There are several lines of scientific evidence demonstrating the role of physical inactivity in the aetiology, as well as the beneficial effects of exercise in both the prevention and treatment of type 2 diabetes and its related morbidity.2 A sedentary person may become even more metabolically unfit over the years; therefore, various forms of physical activity may be necessary to short-circuit unhealthy molecular signals causing metabolic disease.3
Physical effort is an integral part of programs for the prevention and treatment of diseases.4 Health-related physical fitness includes maximal aerobic capacity (VO2 max), muscle strength, flexibility, and body composition parameters. However, daily energy expenditure parameters are closely related to physical fitness. Identifying which health-related physical fitness and energy expenditure parameters are impaired in patients with newly diagnosed type 2 diabetes could contribute to the development of more beneficial physical rehabilitation strategies.
Thus, the aim of this study was to investigate health-related physical fitness parameters, such as maximal aerobic capacity, muscle strength, flexibility, body composition, and body fat distribution, and daily energy expenditure parameters, such as total energy expenditure, daily physical activity and resting metabolic rate (RMR) changes in newly diagnosed type 2 diabetic patients, and compare them with healthy controls.
MATERIALS AND METHODS
This study was designed as a comparative association analysis between patients with type 2 diabetes and healthy controls regarding daily energy expenditure, physical fitness, and anthropometric parameters. The study protocol was approved by the local Ethics Committee of Clinical Research, and all patients and controls participated voluntarily and provided written informed consent.
Subjects
Patients who were referred to the Department of Internal Medicine at the Afyon Kocatepe University Faculty of Medicine were preselected for the study following the diagnosis of type 2 diabetes by a specialist. The patients were considered to have type 2 diabetes mellitus if: the fasting plasma glucose (FPG) level was ≥ 126 mg/dl (7 mmol/l), and/or the second hour plasma glucose level was ≥ 200 mg/dl (11.1 mmol/l) following an oral glucose tolerance test, and/or random plasma glucose level was ≥ 200 mg/dl (11.1 mmol/l), and/or the value of the HbA1c was ≥ 6.5%, in addition to hyperglycaemia symptoms.5 The healthy controls were recruited from voluntary hospital staff and individuals who accompanied relatives during their hospital visits. Inclusion criteria required that patients had newly diagnosed type 2 diabetes, and no exercise-related risks. Exclusion criteria were acute infection, dehydration, cardiopulmonary and renal diseases, malignancy, the use of drugs that might affect heart rate (such as beta blockers), musculoskeletal disorders, psychiatric diseases (such as severe affective disorder and psychosis), and other metabolic diseases. In all, 80 patients (40 male, 40 female) with type 2 diabetes and 80 healthy controls matched for age and body mass index (BMI) (40 male, 40 female) were included in this study. Because of their biological differences, the analysis of the data was performed separately for men and women.
Physiological measurements
Before the exercise test, the risk of exercise for the participants was assessed according to American College of Sports Medicine criteria.6 The maximum volume of oxygen consumed to produce energy was estimated with the Astrand test protocol, a valid submaximal exercise test for estimating VO2 max (aerobic exercise capacity, or cardiorespiratory fitness).7 The Astrand test was performed on a computerised cycle ergometer (Monark 839E, Monark Exercise AB, Vansbro, Sweden). The subjects were asked to perform a six-minute submaximal exercise test, reaching a steady state heart rate.8 VO2 max was expressed in litres per minute VO2 max (l/min) and millilitres per kilogram of body weight VO2 max (ml/kg/min).
Handgrip strength was measured with a digital dynamometer (Grip Strength Dynamometer T.K.K.5401, Takei Co., Tokyo, Japan). The patients were asked to hold the dynamometer parallel to the side of the body and squeeze the handgrip dynamometer as hard as possible while taking care not to hold their breath (Valsalva manoeuver). The test was repeated three times with each hand, and the highest of the three scores was recorded.9 Back-leg strength was determined during the maximal isometric strength of the trunk muscles in the standing posture with 30 degrees of lumbar flexion using a digital back muscle strength meter (Back Strength Dynamometer T.K.K.5402, Takei Co., Tokyo, Japan). Participants were asked to stand upright on the base of the dynamometer with their arms straight and their fingers extended downward as far as possible on the fronts of their thighs. Then the participants bent forward slightly, grasped the bar, and lifted it as high as possible while keeping their legs straight and their feet flat on the base of the dynamometer. The test was repeated three times, and the highest of the three scores was recorded.10
Trunk flexibility was assessed with a digital flexibility testing device (Standing Trunk Flexion meter T.K.K.5403, Takei Co., Tokyo, Japan) after stretching. The participants were asked to stand barefoot on a specially designed measuring bench, placing their toes even with the front edge of the bench. The mobile part of the scale was raised to the uppermost distance (-20 cm). While standing on the bench, participants were asked to bend over and reach down as far as possible, without bouncing, while keeping their knees locked. The test was repeated three times, and the highest of the three scores was recorded.11
Daily physical activity and related parameters were monitored with a metabolic Holter monitor (SenseWear Armband MF-SW, BodyMedia Inc., Pittsburgh, PA, USA) 24 hours per day for seven days. The participants wore the armband over the triceps muscle of the left arm, at the midpoint between the acromion and olecranon.11 The metabolic Holter monitor continuously recorded an array of physiological data from the four sensors contained on the armband (skin temperature, galvanic skin response, heat flux, and near body temperature), as well as by triaxial accelerometry. These physiological data as well as the participant characteristics (including gender, age, height, weight, smoking status, and handedness) were then processed by the manufacturer’s software using advanced algorithms to calculate and report the total energy expenditure, daily number of steps, and daily sleep duration in a free-living environment.
RMR was measured using an indirect calorimeter (Quark b2 , Cosmed, Rome, Italy) with a computerised metabolic card, which analysed oxygen consumption and carbon dioxide production.12 The participants were asked not to eat for 12 hours and not to exercise for 24 hours before the test. After resting for 15 minutes, the measurements were applied to the subjects in a silent, unlit laboratory, which was at room temperature. The participants were asked to put on a face mask, lay in a supine position, and not move their arms or legs during the test.13
Anthropometric measurements
Body composition parameters were determined with a bioelectrical impedance analysis (BIA) system (Bodystat 1500, Bodystat Ltd., Douglas, Isle of Man, UK). The basic premise of the BIA procedure is that the volume of fat-free tissue in the body is proportional to the electrical conductivity of the body.14 Some precautions were taken before the measurements. The participants were instructed to avoid eating or drinking for four hours, using diuretics for seven days, participating in strenuous exercise for 24 hours, and consuming alcohol for 48 hours prior to the test procedure.14,15 The data were analysed using the manufacturer’s software, and body fat percentage and lean body mass were determined for each patient.
Circumference measurements were taken at anatomical positions with a 7 mm wide tape measure. The tape measure was held parallel to the ground and completely surrounded the part of the body, but it did not compress the subcutaneous fat tissue.14 The waist, abdomen, and hip sites, which reflect central obesity, were used for circumference measurements.16 The waist-to-hip ratio was also calculated. BMI was calculated as the body weight divided by the square of the height (kg/m2 ).
Biochemical measurements
The peripheral venous blood samples were taken from all participants in the study after overnight fasting of at least eight hours. The blood samples were centrifuged for 5 minutes at 4000 rpm to obtain the serum samples; while the FPG and lipid levels which contained total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL-C), and triglycerides (TG) were analysed in an autoanalyser (Cobas 6000-c501, Roche Applied Sciences, Basel, Switzerland) using diagnostic kits (Roche Diagnostics, Mannheim, Germany). The HbA1c (glycated haemoglobin) analyses were performed using the electrophoretic method.
Statistical analysis
The data were analysed using the Statistical Package for the Social Sciences (SPSS) version 18.0 software (SPSS Inc., Chicago, IL, USA). The distribution of the group was analysed with the Kolmogorov-Smirnov test. Differences between the groups were determined with either a Student’s t-test or the Mann–Whitney U-test. The correlations between the parameters were analysed with Pearson and Kendall’s tau correlation tests. All parametric results were expressed as mean ± standard deviation, for each group. The significance level was determined as p ≤ 0.05.
RESULTS
In all, 85 participants were invited to participate in the study. Five of them refused because they did not want to perform the exercise and other tests. None of the patients reported any problems during strength or/and aerobic exercise tests. The mean HbA1c (%) values were 9.1±2.7 and 8.1±2.5 in the male and female patients with type 2 diabetes, respectively. The mean values for age, BMI, body fat percentage, VO2 max, strength, flexibility, RMR, daily number of steps, and total energy expenditure for the male and female patients and controls are shown in tables 1 and 2. 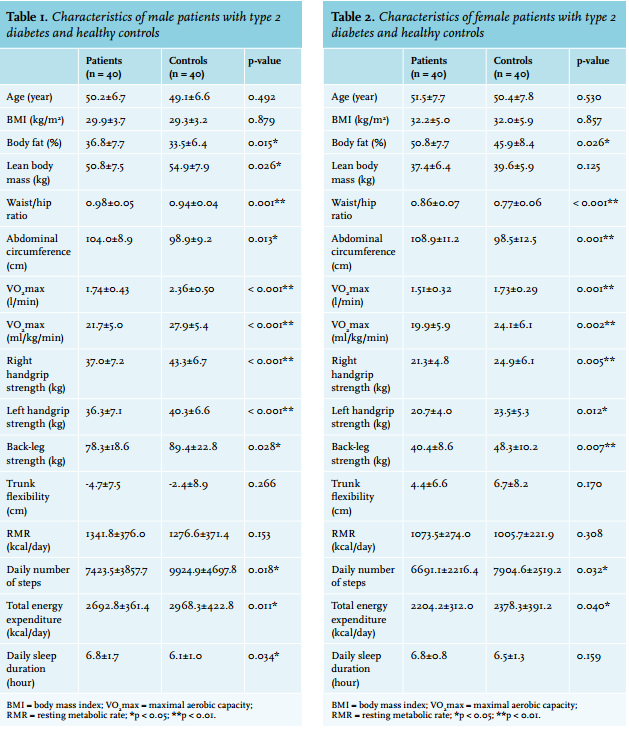
The mean values for the RMR, trunk flexibility, lean body mass, and daily sleep duration did not differ significantly between the female patients and the controls. However, the body fat percentage, waist/hip ratio, and abdominal circumference were significantly higher, whereas the maximal aerobic capacity values (VO2 max [l/min] and VO2 max [ml/kg/min]), all strength measurements, daily number of steps, and total energy expenditure were significantly lower in the female patients than in the controls.
Tables 3 and 4 demonstrate the mean values of the blood pressure, FPG, and lipid profile in the male and female patients with type 2 diabetes and the healthy controls. The mean values for the diastolic blood pressure, TC, HDL-C, LDL-C, VLDL-C, and TG did not differ significantly between the male patients and the controls; nevertheless, the systolic blood pressure and FPG were significantly higher in the male patients, compared with the controls. The mean values for the HDL-C did not differ significantly between the female patients and the controls; however, the systolic and diastolic blood pressures, FPG, TC, LDL-C, VLDL-C, and TG were significantly higher in the female patients, compared with the controls.
Table 5 lists the correlations of FPG with other parameters that were found to be statistically significant in the male and female type 2 diabetic patients. The FPG showed positive correlations with body fat percentage, waist/hip ratio, and daily sleep duration, and negative correlations with maximal aerobic capacity values (VO2 max [l/min] and VO2 max [ml/kg/min]), right handgrip strength, back-leg strength, total energy expenditure, and daily number of steps in male patients. However, the FPG showed positive correlations with systolic and diastolic blood pressures; and negative correlations with the maximal aerobic capacity value (VO2 max [l/min]), right handgrip strength, back-leg strength, and daily number of steps in the female patients.
DISCUSSION
In this cross-sectional study, health-related physical fitness and daily energy expenditure parameters were investigated together and comprehensively in newly diagnosed (not treated) type 2 diabetic patients in both genders. We found that the VO2 max, muscle strength, daily physical activity, total energy expenditure, obesity, and body fat distribution were impaired, and firstly found that the RMR and trunk flexibility were not different in patients with newly diagnosed type 2 diabetes, when compared with the healthy controls. Only the lean body mass was decreased in the male patients compared with the controls. Fagour et al. 17 measured the physical activity levels in outpatients with type 2 diabetes, and age-gendermatched individuals without diabetes using a metabolic Holter monitor. They found that the values for total energy expenditure and daily number of steps were all significantly lower in patients with type 2 diabetes when compared with the controls.17 In other cross-sectional studies, it was found that physical activity was associated with better glycaemic control and the amelioration of some cardiovascular risk factors in patients with type 2 diabetes.18-21 The studies with respect to aerobic exercise intervention in patients with type 2 diabetes showed that the training program resulted in improved glucose tolerance, metabolic control, and management of illness.22,23
Parallel to the literature, we found that the daily step number, VO2 max, and total energy expenditure values were lower in male and female patients than in the controls (tables 1 and 2). The daily sleep duration was significantly higher in male patients, compared with the controls (table 1). In addition, the FPG value was negatively correlated with the daily number of steps and VO2 max values in the patients, in both genders, and negatively correlated with the total energy expenditure value in male patients (table 5). The VO2 max is considered to be the gold standard for the evaluation of cardiorespiratory fitness and/or physical functional capacity,14 and it is most often affected by moderate to vigorous activities, such as sports activities.9
Previous cross-sectional studies have indicated that individuals with type 2 diabetes have a higher RMR.24,25 Conversely, we found no significant difference with regard to the RMR between the patients and controls in either gender (tables 1 and 2). The RMR is the component of energy expenditure that explains the largest proportion (70-80%) of an individual’s total daily energy expenditure.26 Fontvieille et al. 25 suggested that increased basal and sleeping metabolic rates, resulting in increased 24-hour sedentary energy expenditure, may play a role in the weight loss so often observed in type 2 diabetic patients, in addition to the energy loss from glycosuria. The increased RMR in individuals with type 2 diabetes may be a late abnormality, secondary to their metabolic deterioration. Our results revealed that because of reduced physical activity, total energy expenditure was found to be lower in the patients when compared with the controls (tables 1 and 2). We believe that impaired total energy expenditure can be ameliorated by both daily physical activity and aerobic exercise training in newly diagnosed type 2 diabetic patients. The glucose balance is significantly influenced by the physical fitness level, which is likely to be an important element in the pathogenesis of type 2 diabetes, as well as in the treatment of that disease.23 Ozdirenc et al.27 evaluated body composition, cardiopulmonary, musculoskeletal and motor fitness in 30 patients with type 2 diabetes, and 30 healthy non-diabetic controls matched for BMI and age. They found that the body fat percentage was higher, and VO2 max and handgrip strength were lower in the diabetic patients when compared with the control group. In some prospective studies, strength training was proven to be effective with clinical improvements in the glycaemic control and muscle mass.28,29 Nevertheless, Zois et al. 30 found that a combined 16-week strength and aerobic training program could induce positive adaptations in the lipid profile, glycaemic control, insulin resistance, cardiovascular function, and physical fitness in post-menopausal women with type 2 diabetes. In this study, we evaluated right and left handgrip strength and back-leg strength as samples of whole body strength. We found that both the handgrip and back-leg strength values were significantly lower in male and female patients than in the healthy controls (tables 1 and 2). The daily sleep duration was significantly higher, whereas the lean body mass value was significantly lower in male patients compared with the controls (table 1). Further, the FPG value was negatively correlated with the right handgrip strength and back-leg strength values in the patients, in both genders (table 5).
The skeletal muscle mass is a large part of the lean body mass,31 and we believe that impaired aerobic exercise capacity and whole body strength might lead to decreased muscle mass, especially in male type 2 diabetic patients. Both aerobic and resistance training are important for individuals with diabetes and, ideally, a program that combines the two types of training should be undertaken to achieve maximal glycaemic and other benefits.32 The epidemic of overweight and obesity has caused a dramatic increase in the number of individuals with metabolic abnormalities and premature cardiovascular disease.33 Daniele et al.34 found that sedentary diabetic patients had higher waist circumference, waist-to-hip ratios, more depressive symptoms, and worse health-related quality of life. Furthermore, Kim et al. 35 suggested that the BMI and central obesity were good predictors of type 2 diabetes risk in Koreans. Kaizu et al.18 divided a total of 4870 Japanese type 2 diabetic patients into eight groups according to their leisure-time physical activity. They found that leisure-time physical activity was dose-dependently associated with BMI and waist circumference. In a prospective study with respect to blood pressure, it was found that systolic and diastolic blood pressures, as well as heart rate, were significantly decreased by aerobic exercise (three times per week for three years) in patients with type 2 diabetes.36 Similarly, we found that the body fat percentage, waist/hip ratio, abdominal circumference, and systolic blood pressure values were significantly higher in patients, as compared with the controls, in both genders (tables 1 and 2). Additionally, the lean body mass value was significantly lower in male patients, compared with the controls, whereas the diastolic blood pressure value was significantly higher in female patients, compared with the controls (tables 1 and 4).
The FPG value was positively correlated with the body fat percentage and waist/hip ratio in male patients; and positively correlated with the systolic and diastolic blood pressures in female patients (table 5). Although the blood pressure values were found to be significantly different between the patients and controls, the blood pressures were in the normal margin in both groups. The lipid profile was found to be deteriorated in female patients, but no correlation was found between the FPG value and lipid profile parameters (tables 3 and 4). Nevertheless, multiple factors, such as nutritional status, content of food, genetic and environmental factors, and level of physical activity, may change the lipid profile. We believe that physical inactivity might contribute to obesity and central obesity in patients with type 2 diabetes. Therefore, lifestyle changes which promote adherence to habitual physical activity must be included in the management of newly diagnosed type 2 diabetes. These early lifestyle changes may provide numerous benefits, such as the prevention of obesity, metabolic syndrome, and cardiovascular risks through type 2 diabetes.
The lack of a validity study using the Astrand exercise test in type 2 diabetic patients was a limitation of this study. Future studies should investigate the validity of this exercise test in patients with type 2 diabetes. In conclusion, this study revealed that daily physical activity, total energy expenditure, aerobic exercise capacity, muscle strength, obesity, and body fat distribution were impaired in male and female patients with type 2 diabetes, when compared with the healthy controls. In addition, lean body mass was decreased in male patients, compared with the controls, whereas the lipid profile was deteriorated in female patients, as compared with the controls. We found that resting metabolic rate and trunk flexibility values were not significantly different between the patients and the controls in either gender. We suggest that using exercise intervention especially comprised of strength training and aerobic activities, including not only daily slow activities but also moderate to vigorous activities, as a lifestyle modification in newly diagnosed type 2 diabetic patients, might be helpful for the development of more successful illness management strategies, including early intervention.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES